Figures & data
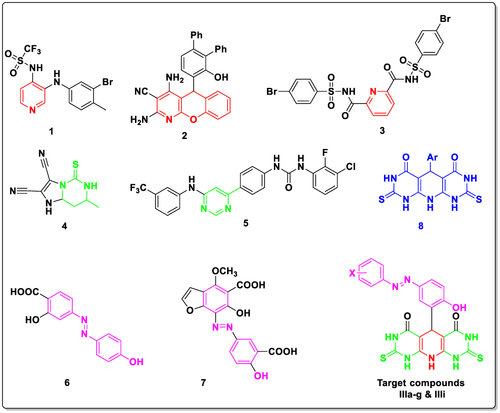
Figure 1 Representative examples of previously identified anti-inflammatory pyridines (1–3), pyrimidines (4, 5), azo containing derivatives (6,7), tricyclic pyridopyrimidine (8), and target compounds IIIa–i.
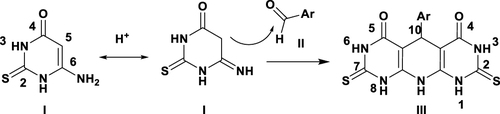
Scheme 1 The synthetic pathway of molecules IIIa–i. Reagents and conditions: a) NaOC2H5, C2H5OH, reflux, 6h, 99%; b) NaNO2, HCl, 0 oC, 2 h; c) Aromatic aldehyde, NaOH, stirring, 0 oC, 12 h; d) conc. HCl, methanol, rt, 7h, 66–93%.
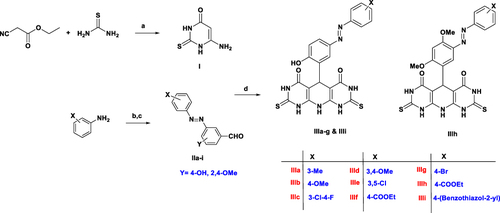
Table 1 NMR Data of the Novel Compounds IIIa-I
Table 2 In vitro COXs Inhibitory Action of Molecules IIIa–i
Table 3 In vivo Anti-Inflammatory Activities of Compounds IIIa–i
Table 4 Gastric Ulcerogenic Effect of Compounds IIIf–h
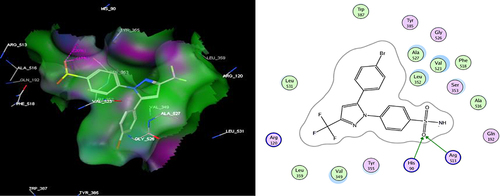
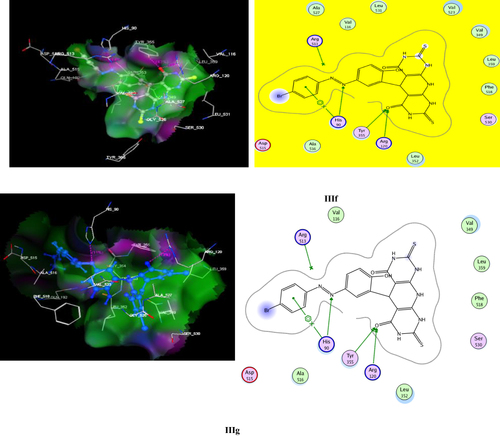
Table 5 The Virtual Docking Data of Compounds IIId, IIIf, IIIg, IIIi and SC-558