Figures & data
Table 1 Cosmetic Use of Various Nano-Delivery Systems and Their BenefitsCitation40
Table 2 Potential Functional Groups Were Identified Using Fourier Transform Infrared Spectrometry (FTIR) in Bulbine Frutescens Extracts and Synthesized Gold Nanoparticles (AuNPs)
Figure 1 (A) Fourier-transform infrared spectrometry (FTIR) of the freeze-dried leaf juice extract (BFE) and synthesized gold nanoparticles (BFEAuNPs), (B) high-resolution transmission electron microscopy (HRTEM) and (C) selected area diffraction pattern (SAED).
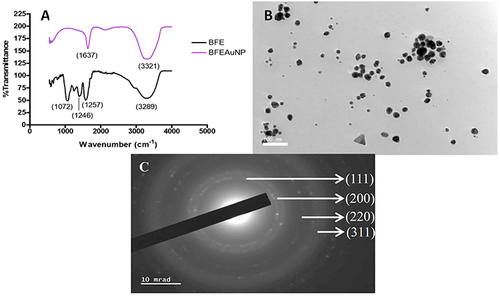
Figure 2 (A) Fourier-transform infrared spectrometry (FTIR) of the ethanolic whole leaf extract (BFE+) and synthesized gold nanoparticles (BFE+AuNPs), (B) high-resolution transmission electron microscopy (HRTEM) and (C) selected area diffraction pattern (SAED).
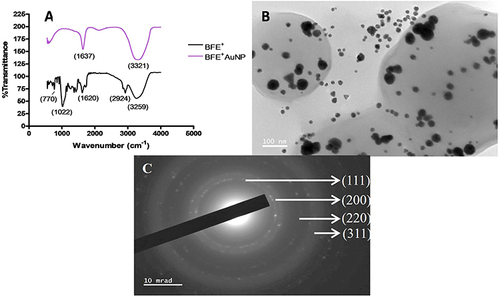
Figure 3 (A) Fourier-transform infrared spectrometry (FTIR) of the gel extract (BFG) and synthesized gold nanoparticles (BFGAuNPs), (B) high-resolution transmission electron microscopy (HRTEM) and (C) selected area diffraction pattern (SAED).
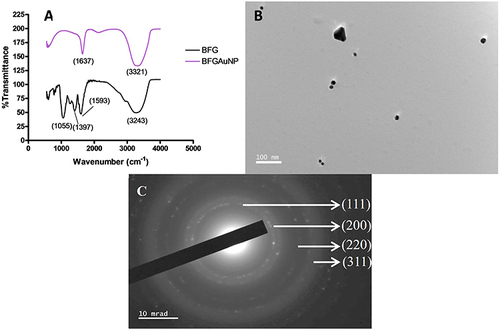
Figure 4 (A) Fourier-transform infrared spectrometry (FTIR) of the preserved leaf juice solution (BFS) and synthesized gold nanoparticles (BFSAuNPs), (B) high-resolution transmission electron microscopy (HRTEM) and (C) selected area diffraction pattern (SAED).
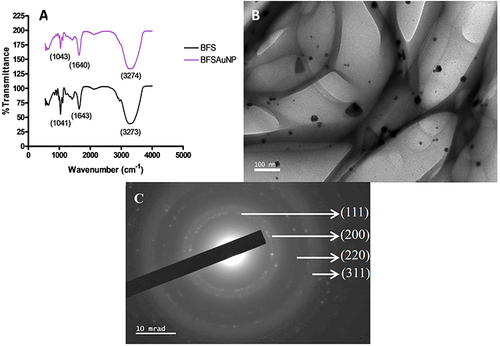
Figure 5 In vitro stability of Bulbine frutescens freeze-dried leaf juice synthesized gold nanoparticles (BFEAuNPs) in (A) Roswell Park Memorial Institution (RPMI-1640) media, (B) Dulbecco’s modified Eagle’s media (DMEM), (C) 5% sodium chloride (NaCl), (D) pH of 4, (E) 7 and (F) 10 and (G) 0.5% bovine serum albumin (BSA).
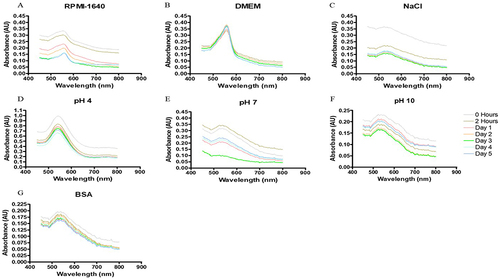
Figure 6 In vitro stability of Bulbine frutescens ethanolic whole leaf synthesized gold nanoparticles (BFE+AuNPs) in (A) Roswell Park Memorial Institution (RPMI-1640) media, (B) Dulbecco’s modified Eagle’s media (DMEM), (C) 5% sodium chloride (NaCl), (D) pH of 4, (E) 7 and (F) 10 and (G) 0.5% bovine serum albumin (BSA).
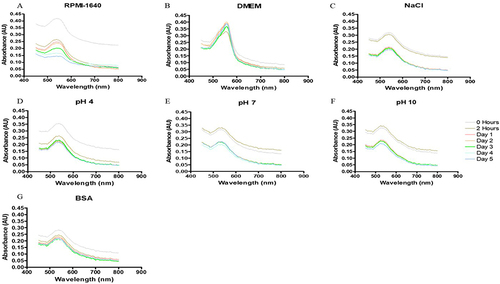
Figure 7 In vitro stability of Bulbine frutescens gel synthesized gold nanoparticles (BFGAuNPs) in (A) Roswell Park Memorial Institution (RPMI-1640) media, (B) Dulbecco’s modified Eagle’s media (DMEM), (C) 5% sodium chloride (NaCl), (D) pH of 4, (E) 7 and (F) 10 and (G) 0.5% bovine serum albumin (BSA).
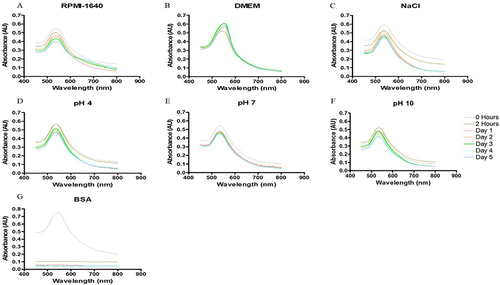
Figure 8 In vitro stability of Bulbine frutescens preserved leaf juice synthesized gold nanoparticles (BFSAuNPs) in (A) Roswell Park Memorial Institution (RPMI-1640) media, (B) Dulbecco’s modified Eagle’s media (DMEM), (C) 5% sodium chloride (NaCl), (D) pH of 4, (E) 7 and (F) 10 and (G) 0.5% bovine serum albumin (BSA).
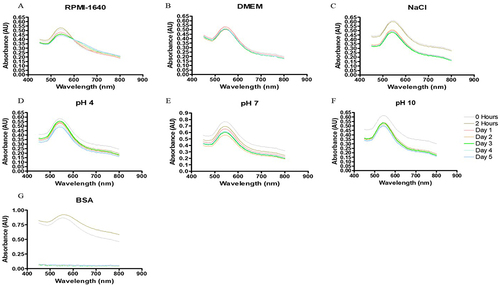
Figure 9 Cell viability of (A) BF freeze-dried leaf juice (BFE), ethanolic whole leaf (BFE+) and gel extract (BFG), (B) BF freeze-dried leaf juice synthesized gold nanoparticle (BFEAuNPs) and ethanolic whole leaf synthesized gold nanoparticle (BFE+AuNPs) solutions at a concentration of 400–50 µg/mL and (C) BF preserved leaf juice (BFS), preserved leaf juice synthesized gold nanoparticle (BFSAuNPs) solutions at a concentration of 2 and 1% and gel synthesized gold nanoparticle (BFGAuNPs) solution at 10 and 5% on wound stimulated human keratinocyte (HaCaT) cells. Data represents mean ± SEM (n=2). A significant difference was determined using a one-way ANOVA followed by Dunnett’s multiple comparison test, where p < 0.01 (**) and p < 0.001 (***) indicate significance when compared to the vehicle (0.5% DMSO) and water control (+).
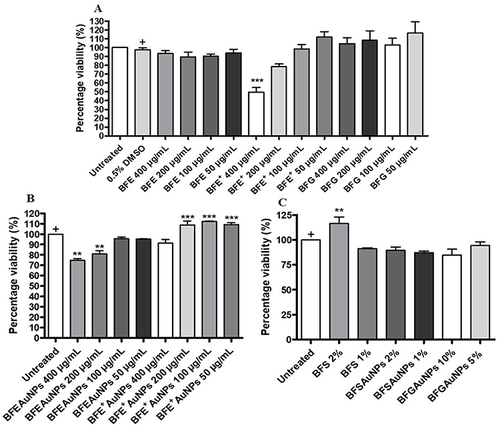
Figure 10 Percentage wound closure of (A) BF freeze-dried leaf juice (BFE), ethanolic whole leaf (BFE+) and gel extract (BFG) at 100 and 50 µg/mL, (B) BF freeze-dried leaf juice synthesized gold nanoparticle (BFEAuNPs) and ethanolic whole leaf synthesized gold nanoparticle (BFE+AuNPs) solutions at 100 and 50 µg/mL and (C) BF preserved leaf juice (BFS), preserved leaf juice synthesized gold nanoparticles (BFSAuNPs) at 2 and 1% and gel synthesized gold nanoparticles (BFGAuNPs) at 10 and 5% on human keratinocyte (HaCaT) cells. Data is represented as mean ± SEM (n=2) Significant difference was determined using a one-way ANOVA followed by Dunnett’s multiple comparison test, where p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) indicate significance when compared to the vehicle (0.5% DMSO) control or the untreated control (+).
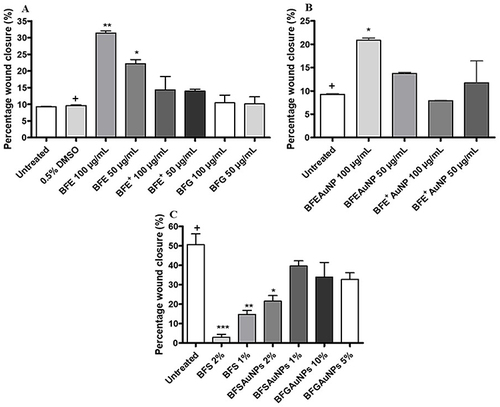
Figure 11 Cell viability of the freeze-dried leaf juice (BFE) extract and synthesized gold nanoparticles using the freeze-dried leaf juice (BFEAuNPs) at a concentration of 200, 100 and 50 µg/mL on phorbol 12-myristate 13-acetate (PMA) stimulated granulocytes. Data represents mean ± SEM (n=2). A significant difference was determined using a one-way ANOVA followed by Dunnett’s multiple comparison test when compared to either the untreated (media with PMA) or 0.25% DMSO (vehicle) control (+).
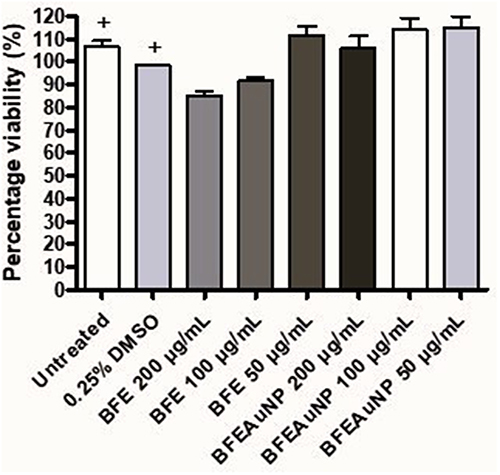
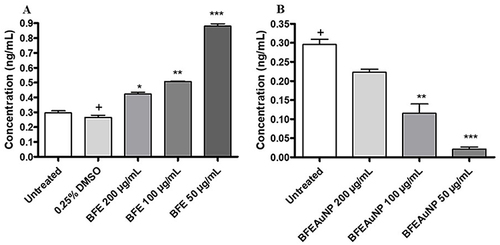
Figure 12 Histamine production of (A) BF freeze-dried leaf juice extracts (BFE) and (B) BF freeze-dried leaf juice synthesized gold nanoparticles (BFEAuNPs) at a concentration of 200, 100 and 50 µg/mL using phorbol 12-myristate 13-acetate (PMA) stimulated granulocytes. Data represents mean ± SEM (n=2). Significant difference was determined using a one-way ANOVA followed by Dunnett’s multiple comparison test, where p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) indicate significance when compared to either the 0.25% DMSO (vehicle) or untreated (media) control (+).