Figures & data
Figure 1 Schematic diagram of the subunits of collagen VI. All the 6 chains and their encoding genes are presented. The molecular structures of collagen VI subunits are similar: a short collagenous triple-helical domain and three von Willebrand factor type A (vWF-A) domains (N1, C1, and C2). Nine additional vWF-A domains are found in the N-terminal domain (N2–N10) and 3 special domains are found in the C-terminal domain (C3–C5) in the collagen alpha-3 (VI) chain. The area marked by arrows shows the collagenous domain and the cleaved C5 domain fragment endotrophin. In humans, the COL6A4 gene is disrupted by a chromosome break to create two pseudogenes. In addition, the N2–N10 vWF-A domains are each encoded by a single exon accordingly from exon 11 to exon 3 (E11–E3), and the domains undergo alternative splicing are marked orange. Due to the alternative splicing of COL6A3 at the pre-mRNA level, the N3, N5, N7, N9 and N10 vWF-A domains of the collagen alpha-3 (VI) chain are either present or absent in the final chain. The tumor-specific alternative splicing of exons 6, 4, and 3 (E6, E4, and E3) are also showed in the Figure in Red marks. This figure was modified with permission from Cescon M, Gattazzo F, Chen P, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128(19):3525–3531. doi:10.1242/jcs.169748. Copyright 2015, The Company of Biologists Ltd.Citation100
Abbreviations: TH, triple helical; vWF-A, von Willebrand factor type A; E3, exon 3; E4, exon 4; E5, exon 5; E6, exon 6; E7, exon 7; E8, exon 8; E9, exon 9; E10, exon 10; E11, exon 11.
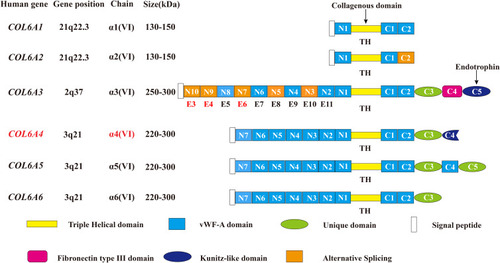
Figure 2 Schematic diagram of collagen VI assembly process. Collagen VI is firstly assembled into a triple helix monomer by the alpha-1 (VI), alpha-2 (VI) and alpha-3 (VI) chains in a stoichiometric ratio of 1:1:1. Then the triple helix monomers are staggered into anti-parallel dimers bonded by disulfide bonds, and the anti-parallel dimers are also aligned through disulfide bonds to form tetramer. Further, these tetramers are secreted to the extracellular matrix and form beaded microfibrils in a way of end-to-end connection of overlapped N-terminal globular domains through non-covalent bonds. The maturation of collagen VI microfibrils (fibril processing) in the extracellular matrix is a continuous process that starts immediately when tetramers are secreted, and requires proteolytic process involving the removal of the C-terminal domains of the collagen alpha-3 (VI) chain (for example, the C5 or the C2–C5 domains cleavage fragments) after microfibril assembly. The alpha-5 (VI) and alpha-6 (VI) chains can be substituted for alpha-3 (VI) chains in human. Furin-like PPC and BMP-1 are two proteins involving the endotrophin-containing fragments releasing process, and the cleavage sites are between the C4 and C5 domains as well as the the C1 and C2 domains. This figure was modified with permission from Furthmayr H, Wiedemann H, Timpl R, Odermatt E, Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983;211(2):303–311. doi:10.1042/bj2110303. Copyright 1983, Portland Press, Ltd.Citation101
Abbreviations: vWF-A, von Willebrand factor type A; Furin-like PPC, furin-like proprotein convertase; BMP-1, bone morphogenetic protein 1.
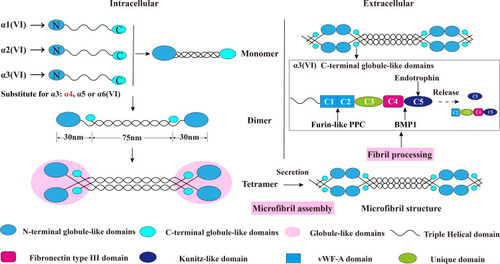
Table 1 Collagen Alpha-3 (VI) Chain and Endotrophin Exist in Different Types of Cancer
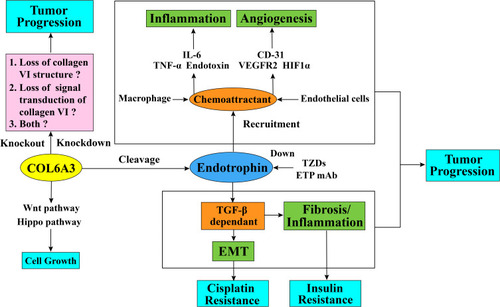
Figure 3 Schematic diagram of collagen alpha-3 (VI) chains and endotrophin function in tumor progression and cisplatin resistance. The collagen alpha-3 (VI) chain C5 domain fragment endotrophin regulates tumor EMT and fibrosis through a TGF-β-dependent pathway. Endotrophin can also promote tumor inflammation through the recruitment of macrophages and enhances the expression level of inflammatory cytokines, such as IL6 and TNF-α, or through the recruitment of endothelial cells into the tumor microenvironment to promote tumor angiogenesis and increase the expression level of angiogenesis markers, such as CD31, VEGFR2, and HIF1α. It is not clear whether the effect of collagen alpha-3 (VI) chain on tumor behavior is by affecting the collagen VI structure stability, either by affecting the signal transduction ability of collagen VI molecules, or both. In terms of drug resistance, endotrophin can induce tumor cells to resist cisplatin through the TGF-β-EMT pathway, and treatment with endotrophin mAb or TZDs can block this process by downregulating endotrophin levels and eventually give rise to increased cisplatin sensitivity. Additionally, COL6A3 is highly expressed in densely growing mouse embryonic fibroblasts (MEFs) and promotes the 3D growth of MEFs by suppressing the Hippo signaling pathway and promoting the Wnt signaling pathway.
Abbreviations: TGF-β, transforming growth factor beta; EMT, epithelial–mesenchymal transition; IL-6, interleukin 6; TNF-α, tumor necrosis factor alpha; CD-31, cluster of differentiation 31; VEGFR2, vascular endothelial growth factor receptor 2; HIF1α, hypoxia-inducible factor 1α; ETP, endotrophin; mAb, monoclonal antibodies; TZDs, thiazolidinediones.