Figures & data
Table 1 Clinical Presentation and Treatment Summary
Table 2 Molecular Profiling of Pre-Pembrolizumab Tumor Biopsy
Figure 1 Clinical and Radiologic Response to pembrolizumab; Immunohistochemistry. (A and B) Clinical response to pembrolizumab; (A) Fungating left groin tumor and satellite nodule at baseline (September 2018), prior to starting pembrolizumab (horizontal red arrows); (B) Dramatic clinical response after 3 cycles of pembrolizumab in primary tumor and adjacent satellite nodule (December 2018) (horizontal red arrows). (C and D) Radiologic response to pembrolizumab; (C) Baseline PET-CT showing fungating and intensely FDG-avid mass (SUVmax 19.6) at the left groin (vertical white arrow) with invasion of the adductor compartment of the left thigh; (D) Follow-up PET-CT after 6 months showing marked reduction in size and metabolic uptake (SUVmax = 3.9) of the tumor (vertical white arrow). (E) Hematoxylin and eosin x20 stain confirming clear cell carcinoma: the tumor is composed of nests of cells with round nuclei, prominent nucleoli and ample clear to eosinophilic cytoplasm; (F) Immunohistochemistry for PD-L1 using the Dako 22C3 pharmDx assay, combined positive score of 45.
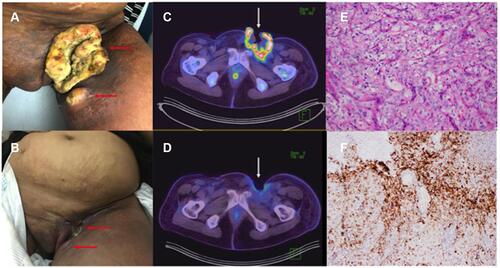
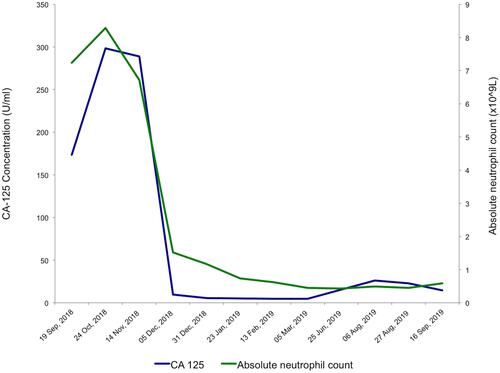
Figure 2 Relevant Biochemistry and Hematology Indices during pembrolizumab treatment. Rapid reduction in serum CA-125 was observed after commencing pembrolizumab in October 2018, with CA-125 dropping precipitously from a baseline of 298 U/mL to 9.5 U/mL in December 2018, after only 3 cycles of pembrolizumab. CA-125 reached a nadir of 4.6 U/mL in February 2019. Gradual increase in CA-125 coincided with clinical disease progression in June 2019. Worsening neutropenia occurred during the course of pembrolizumab, due to underlying myelodysplastic syndrome, which was later confirmed on bone marrow examination.