Figures & data
Table 1 IC50 values determined by receptor phosphorylation assays
Figure 2 Mean steady-state plasma concentrations on day 15 of dosing (maximum concentration versus dose).
Abbreviations: bid, twice daily; qd, once daily; Cmax, maximum concentration.
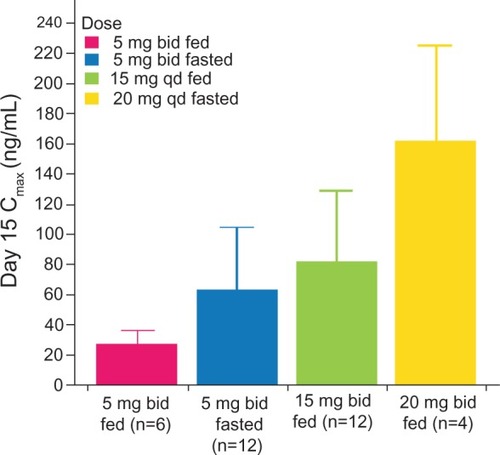
Table 2 Main CYP 3A4 inducers
Figure 3 Maximum percentage tumor decrease of target lesions by Response Evaluation Criteria In Solid Tumors.
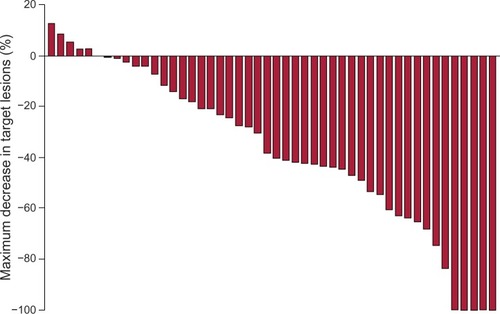
Figure 4 Kaplan–Meier estimated median progression-free survival in patients who received axitinib or sorafenib as second-line therapy for metastatic renal cell cancer. (A) All patients, (B) patients previously treated with cytokine-based regimen, and (C) patients previously treated with sunitinib-based regimen (full analysis set, by independent review committee assessment).
Abbreviations: HR, hazard ratio; PFS, progression-free survival; CI, confidence interval.
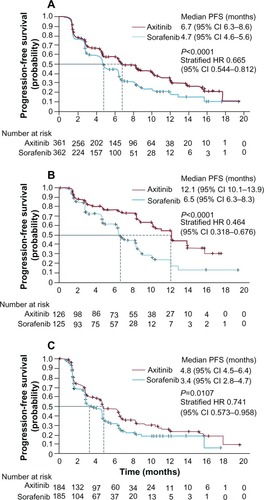
Table 3 Treatment-related adverse events in AGiLe 1046