Figures & data
Figure 1 Schema outlining the activation of the human epidermal growth-factor receptor 2 pathway and antibody blockade by trastuzumab and pertuzumab.
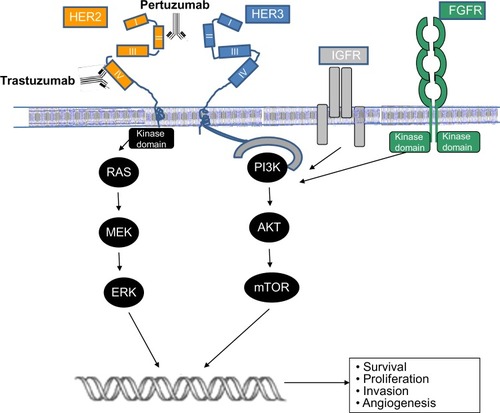
Table 1 Key randomized clinical trials evaluating pertuzumab therapy for HER2-positive breast cancer
Figure 2 Schema of the NeoSphere clinical trial comparing various neoadjuvant HER2-directed therapies for localized HER2-positive breast cancer.
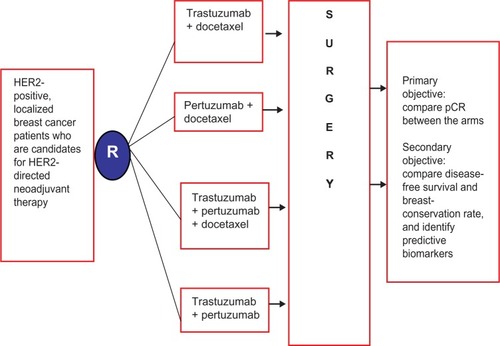
Figure 3 Schema of the APHINITY clinical trial evaluating efficacy of adjuvant pertuzumab based therapy in localized HER2-positive breast cancer.
Abbreviations: HER, human epidermal growth-factor receptor; IV, intravenous; q3 weeks, every 3 weeks; APHINITY, Adjuvant Pertuzumab and Herceptin in Initial Therapy of Breast Cancer.
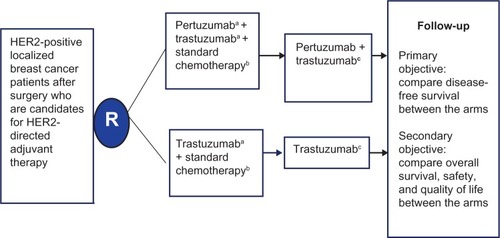
Table 2 Pertuzumab-based chemotherapy regimens as per the US Food and Drug Administration prescribing label (updated September 2013)
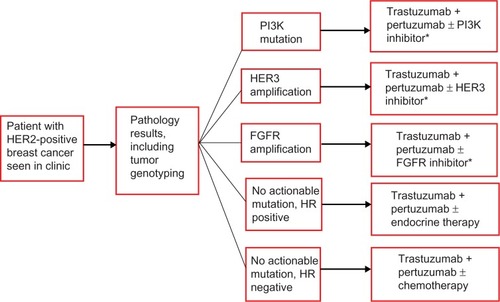
Figure 4 Schema of personalized therapy selection based on molecular profiling of breast cancer.
Abbreviations: PI3K, phosphoinositide 3-kinase; HER, human epidermal growth-factor receptor; FGFR, fibroblast growth-factor receptor; HR, hormone receptor.