Figures & data
Figure 1 Flow diagrams illustrating respondent numbers in each stage of the study.
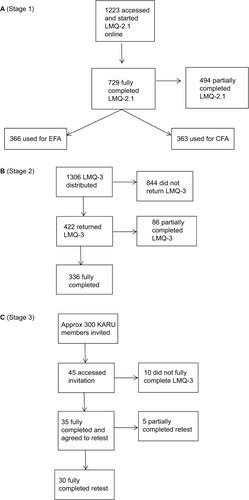
Table 1 Characteristics of study participants
Table 2 EFA-derived factor structure of the LMQ-3 (N=366)
Figure 2 Hierarchical CFA model for the 41-item Living with Medicines Questionnaire version 3.
Abbreviations: CFA, confirmatory factor analyses; Int, interferences with day-to-day life; SideE, side effects; Conc, general concerns about medicines; Effec, lack of effectiveness; Prac, practical difficulties; Relat, patient–doctor relationships and communication about medicines; Cost, cost-related burden; Auto, lack of autonomy/control over medicine use; e1–e41 = variance associated each item; eI–eA represent variance associated with each of the eight factors or domains (interferences to autonomy respectively); Q, question.
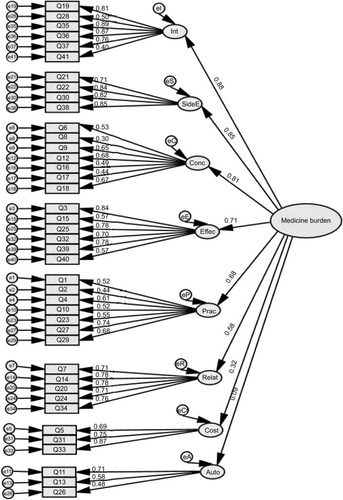
Table 3 Correlations between domains of the LMQ-3 and TSQM-II subscales
Table 4 Known-groups validity of the LMQ-3
Figure 3 Number of missing responses to LMQ questions.
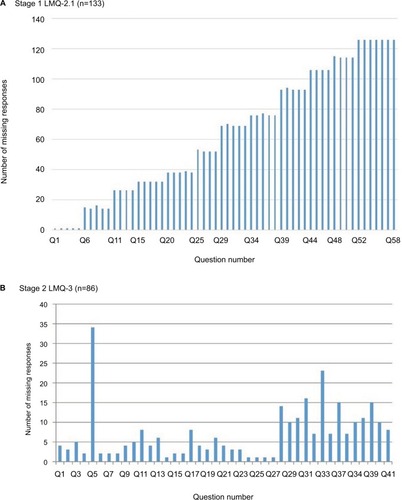
Table 5 Items with more than 2% missing data in Stage 2
Table 6 Differences between full and partial respondents of LMQ-3 in Stage 2
