Figures & data
Figure 1 Trial designs of NAVIGATE and AMAGINE 2/3. In AMAGINE-2 and −3, at week 12, patients who were originally randomized to brodalumab underwent repeat randomization (2:2:2:1) to one of four maintenance regimens: brodalumab at 210 mg every 2 weeks, 140 mg every 2 weeks, 140 mg every 4 weeks, or 140 mg every 8 weeks. Patients who were originally randomized to placebo were switched to brodalumab at a dose of 210 mg every 2 weeks. Patients who were originally randomly assigned to receive ustekinumab and had an adequate response continued to receive ustekinumab every 12 weeks until week 52. These details are reported in more detail in Lebwohl et al 2015.
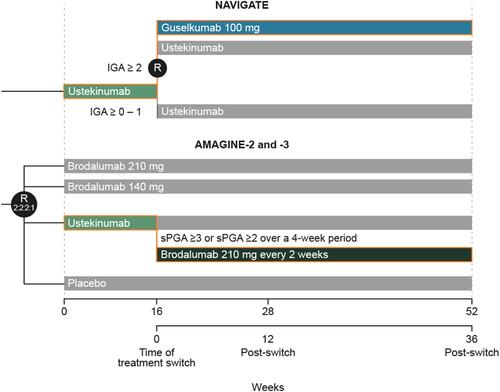
Table 1 Baseline Characteristics of Patients
Table 2 PASI 90, PASI 100, PGA and DLQI Rates with Brodalumab and Guselkumab After Switching from Ustekinumab
Figure 2 Forest plot of PASI 90, PASI 100, PGA success and DLQI 0/1 rates at 12 and 36 weeks after switching from ustekinumab.
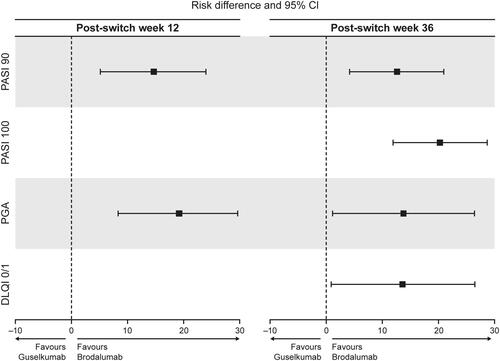
Table 3 PASI 90, PASI 100, and PGA Rates According to PGA Score at Time of Switching from Ustekinumab to Brodalumab
