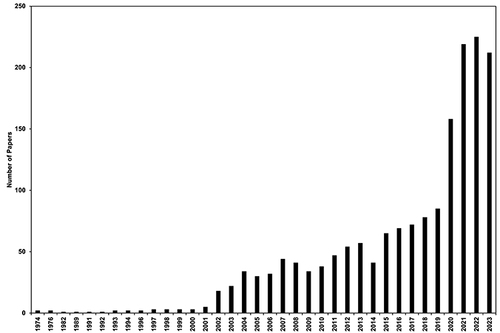
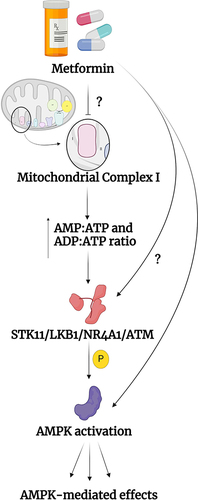
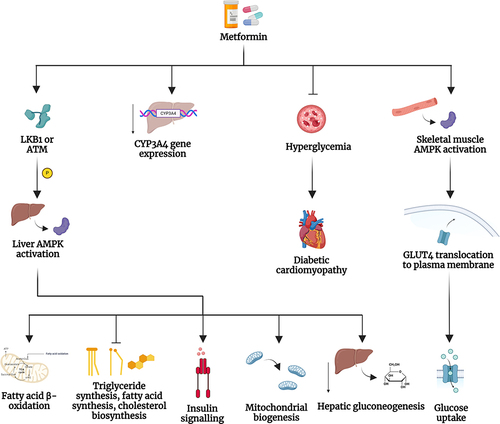
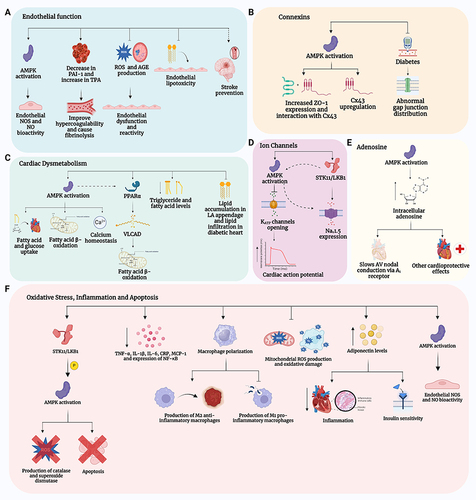
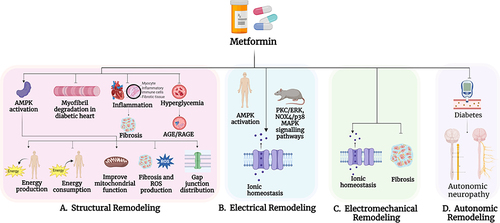
Notes: (
A) (Endothelial function): Metformin, via activation of adenosine monophosphate-activated protein kinase (AMPK) increases the bioactivity of endothelial nitric oxide synthase (eNOS) thereby increasing the generation of nitric oxide (NO). Metformin also reduces hypercoagulability and facilitates fibrinolysis by decreasing plasminogen activator inhibitor-1 (PAI-1) and increasing Tissue Plasminogen Activator (TPA). Additionally, metformin reduces endothelial dysfunction by inhibiting the production of Reactive Oxygen Species (ROS) and Advanced Glycation End-Products (AGE), as well as endothelial lipotoxicity. Lastly, metformin, in part via its vascular protective effects, reduces the risk of stroke. (
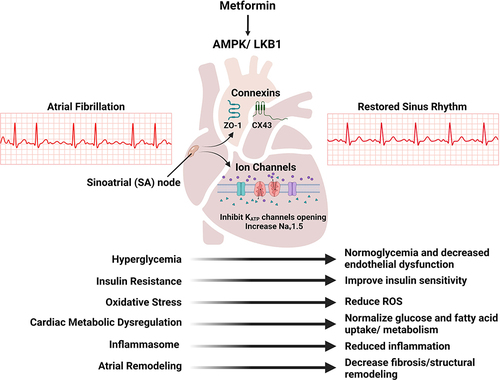
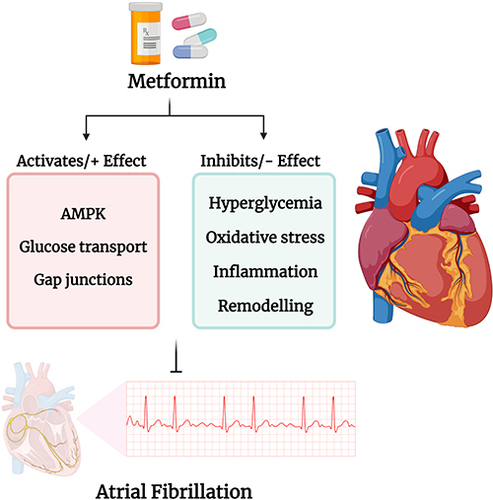
B) (Connexins): Metformin activation AMPK which results not only in beneficial effects on glucose regulation and insulin resistance but also results in increase in the expression of Zonula Occludens-1 (ZO-1) and its interaction with and expression of connexin 43 (Cx43). (
C) (Cardiac Dysmetabolism): Metformin, via its activation of AMPK, increases fatty acid and glucose uptake and fatty acid β-oxidation, while improving calcium homeostasis. Metformin also activates Peroxisome Proliferator-Activated Receptor α (PPARα) either directly or through AMPK, to increase fatty acid β-oxidation by increasing the activity of Very Long-Chain Acyl-Coenzyme A Dehydrogenase (VLCAD). In addition, metformin reduces triglyceride and fatty acid levels in the body and inhibits the accumulation of lipids in the left atrial (LA) appendage and the infiltration of lipids in the diabetic heart. (
D) (Ion Channels): Metformin activation of AMPK results in the inhibition of the opening of ATP-sensitive potassium (K
ATP) channels, and it also increases the expression of voltage-gated sodium channels (Na
v1.5) by activating serine/threonine kinase 11 (STK11/LKB11) (also known as liver kinase B1 (LKB1)), either through AMPK activation or its direct effect on Na
v1.5 expression.
Citation35 (
E) (Adenosine): Metformin activation of AMPK leads to an increase in intracellular adenosine which has cardioprotective effects via the adenosine A
1 receptor. (
F) (Oxidative Stress, Inflammation and Apoptosis): Metformin activation of AMPK via the STK11/LKB1 pathway results in the activation of eNOS, and increased production of catalase and superoxide dismutase which reduces ROS, and inhibits apoptosis. Metformin also decreases the generation of pro-inflammatory cytokines and inflammatory including Tumor Necrosis Factor-α (TNF- α), Interleukin-1β (IL-1β), Interleukin-6 (IL-6), C-Reactive Protein (CRP), Monocyte Chemoattractant Protein-1 (MCP-1), and Nuclear Factor-κB (NF-κB) and induces the polarization of macrophages towards anti-inflammatory M2 subtype over the pro-inflammatory M1 subtype).
Citation35,Citation105,Citation175 Created with BioRender.com.