Figures & data
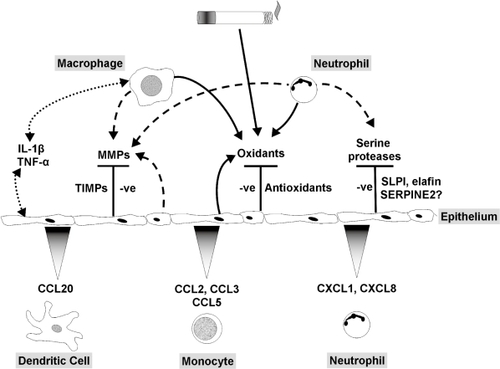
Figure 1 (A) Enzymatic clearance of reactive oxygen species. Superoxide anions undergo dismutation by superoxide dismutase (SOD) leading to the generation of hydrogen peroxide. This in turn is processed by catalase and glutathione peroxidase (GPx). (B) Reduced glutathione (GSH) is regenerated from its oxidized form (GSSG), using NADPH, by glutathione reductase (GRx).
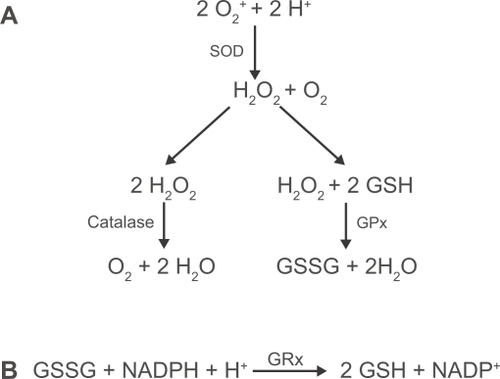
Figure 2 Signaling pathways initiated by TLR-2 and TLR-4 activation. Upon ligation of the TLR-4 receptor complex by exogenous (eg, LPS) or endogenous (eg, hyaluronic acid and β-defensin 2) ligands, MyD88-dependent and MyD88-independent signaling pathways are initiated leading to activation of the transcription factors NF-κB and IRF3. TLR-2 heterodimerises with either TLR-1 or -6 in order to recognise distinct groups of exogenous ligands and signal through a MyD88-dependent pathway. TLR-1/2 recognizes triacyalted (TriAc) lipoproteins and lipoarabinomannan whereas TLR-2/6 recognizes diacylated (DiAc) lipoproteins, lipoteichoic acid and zymosan. TLR-2 may also initiate responses to endogenous products released by necrotic cells.
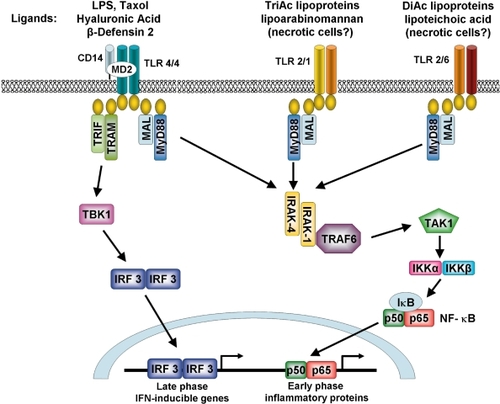
Table 1 Cytokines and chemokines implicated in the pathogenesis of COPD
Figure 3 Cigarette smoke-induced secretion of mucins. Mucin secretion is stimulated following cigarette smoke-induced activation of NADPH oxidase and TACE. NADPH oxidase generates intracellular reactive oxygen species leading to activation of the transcription factor AP-1. TACE cleaves pro-TGFα to generate the active ligand which initiates signaling through the ErbB receptor complex leading to activation of the transcription factors AP-1 and Sp1. Diagram a kind gift of Samir Nuseibeh.
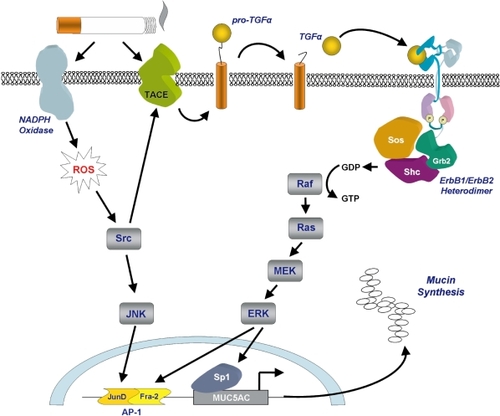
Figure 4 Role of the pulmonary epithelium in cigarette smoke-induced inflammation. The pulmonary epithelium combats leukocyte-derived oxidants and free radicals in cigarette smoke via release of antioxidants. Serine proteases are blocked by low molecular weight inhibitors, SERPINS and TIMPs. Induction of cytokine release by macrophages and epithelial cells and autocrine/paracrine activation of the epithelium stimulates chemokine release and recruitment of monocytes, neutrophils and dendritic cells. In COPD the epithelial defense mechanisms are overwhelmed, leading to increased oxidative stress and proteolytic load, together with leukocyte recruitment, resulting in a chronic cycle of inflammation that may be independent of cigarette smoke exposure.