Figures & data
Figure 1 Schematic outline of the principal components of GH regulation, ie, somatostatin, GHRH, GHRPs (including ghrelin) and IGF-I. Circulating IGF-I is derived mainly from the liver and from other organs to a lesser extend. Positive stimulation and feedback are indicated by the + symbol, and inhibition or negative feedback by the – symbol.
Abbreviations: GHRH, hypothalamic stimulatory growth hormone-releasing hormone; GHRPs, GH- releasing peptides; IGF-1, insulin-like growth factor-I.
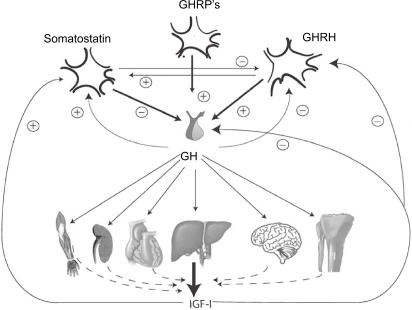
Figure 2 Serum GH concentrations obtained by 10 min blood sampling for 24 hours in a female patient with acromegaly and an age- and gender-matched healthy control subject. Note the difference in y-scale. In the patient, GH secretion is characterized by the increased non-pulsatile component, increased pulsatile secretion, and burst frequency.
Abbreviation: GH, growth hormone.
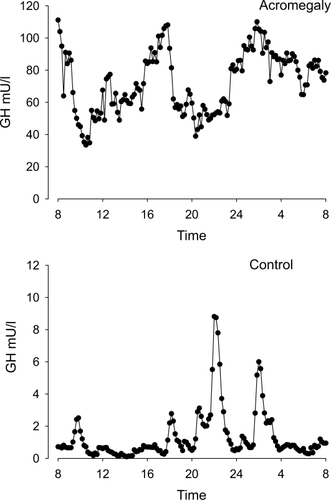
Figure 3 Crystal structure of growth hormone complexed to the extracellular domains of its receptor dimer. The binding sites are indicated by arrows (crystal structure determined by Citationde Vos et al 1992, Protein Database code 3HHR, downloaded graph modified in Chimera UCSF).
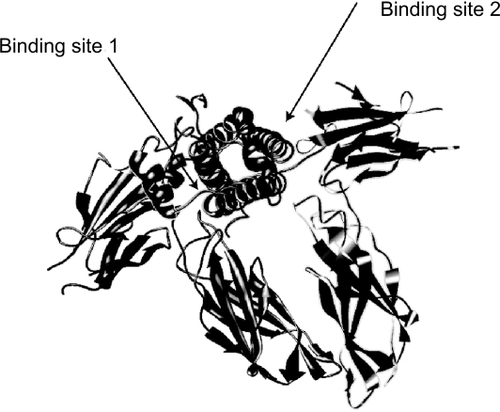
Figure 4 Interaction of GH, B2036, and B2036/PEG (pegvisomant) with the GH receptor. A: GH binds to GH binding protein (GHBP). Binding to its cell surface receptor through sites 1 and 2 induces a change in conformation of the receptor leading to activation and subsequent signaling. B: the site 2 mutation (x) prevents the conformational change needed for activation. C: B2036-PEG binds to GHBP via site 1, which is protected from pegylation by mutation of lysines. High dose of B2036-PEG is required to overcome the steric hindrance by pegylation. Modified after CitationRoss RJ, Leung KC, Maamra M, et al 2001. Binding and functional studies with the growth hormone receptor antagonist B2056-PEG (pegvisomant) reveal effects of pegylation and evidence that it binds to a receptor dimmer. J Clin Endocrinol Metab, 86:1716–23. Copyright © 2001, with permission from the Endocrine Society.
Figure 5 Schematic representation of pegvisomant and the attached PEG polymers. The amino acid substitutions were introduced at positions G120R (binding site 2) and at H18D, H21N, R167N, K168A, D171S, K172R, E174S, and I179T (binding site 1). The mutated amino acids are hatched. Two disulfide bridges between amino acids 53 and 165 and between 182 and 189 are shown by solid lines.
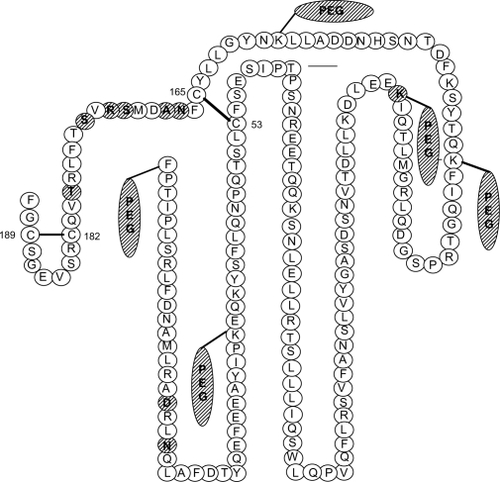
Table 1 Effect of pegvisomant treatment on IGF-I normalization in acromegaly
Table 2 Effect of pegvisomant treatment on metabolic parameters in acromegaly