Figures & data
(A) Timeline of brain development (center) indicating periods (shaded boxes above and below line) when some of the structures discussed in the review are formed as well as when major cellular movements or differentiation events occur (bent arrows). (B) Closure of the neural tube at embryonic day 21–28. The neural folds close over by region (numbered 1–5): failure to close off the region in red will, much later in development, lead to the indicated neural tube defect. (C) Primary vesicle formation commences around the 4th week of gestation, generating the fore-, mid- and hind-brain regions. (D) Histogenesis of the neural tube. Neural progenitor cells undergo mass self-replication prior to migration and differentiation into non-mitotic neurons by the second trimester. Neural progenitor cells with proliferative capacity remain on the V of the neural tube, but differentiated non-mitotic daughter cells migrate outwards (denoted by arrow) along glial processes in the I to form a M distal to the vesicle. (E) Formation of the cerebellum occurs largely in the second semester via a unique morphogenetic event, evaginating from the underlying ependymal tissue. Migration of EGL cells across the surface of the tissue (arrowed at bottom), gives rise to a second distal layer of proliferative cells distal to the vesicle, which produces differentiated cells of the marginal zone. Inside a layer of differentiated and highly-branched P, neurons migrating outwards from the vesicular side (now called the E) form a second, IGL. (F) In the cerebral cortex migration outwards of the differentiated neurons occurs in waves, traveling successively shorter distances, giving rise to the six layers of the cortical plate. Externally (below), this causes the initially smooth surface of the cortex to become more invaginated and folded into a characteristic pattern of gyri and sulci. (G) Maturation of the cortex entails demarcation of regions associated with higher cognitive functions such as language (e.g., Broca’s and Wernicke’s areas) and social interaction (orbitofrontal). Components of the limbic system underneath (dashed lines) also play important roles in sociocognitive functioning.
E: Ependymal layer; EGL: External granular layer; I: Intermediate zone; IGL: Internal granular layer; M: Marginal layer; P: Purkinje cells; V: Ventricular side.
Elements redrawn with permission from [Citation19,Citation20].
![Figure 1. Relevant milestones in human brain development. (A) Timeline of brain development (center) indicating periods (shaded boxes above and below line) when some of the structures discussed in the review are formed as well as when major cellular movements or differentiation events occur (bent arrows). (B) Closure of the neural tube at embryonic day 21–28. The neural folds close over by region (numbered 1–5): failure to close off the region in red will, much later in development, lead to the indicated neural tube defect. (C) Primary vesicle formation commences around the 4th week of gestation, generating the fore-, mid- and hind-brain regions. (D) Histogenesis of the neural tube. Neural progenitor cells undergo mass self-replication prior to migration and differentiation into non-mitotic neurons by the second trimester. Neural progenitor cells with proliferative capacity remain on the V of the neural tube, but differentiated non-mitotic daughter cells migrate outwards (denoted by arrow) along glial processes in the I to form a M distal to the vesicle. (E) Formation of the cerebellum occurs largely in the second semester via a unique morphogenetic event, evaginating from the underlying ependymal tissue. Migration of EGL cells across the surface of the tissue (arrowed at bottom), gives rise to a second distal layer of proliferative cells distal to the vesicle, which produces differentiated cells of the marginal zone. Inside a layer of differentiated and highly-branched P, neurons migrating outwards from the vesicular side (now called the E) form a second, IGL. (F) In the cerebral cortex migration outwards of the differentiated neurons occurs in waves, traveling successively shorter distances, giving rise to the six layers of the cortical plate. Externally (below), this causes the initially smooth surface of the cortex to become more invaginated and folded into a characteristic pattern of gyri and sulci. (G) Maturation of the cortex entails demarcation of regions associated with higher cognitive functions such as language (e.g., Broca’s and Wernicke’s areas) and social interaction (orbitofrontal). Components of the limbic system underneath (dashed lines) also play important roles in sociocognitive functioning.E: Ependymal layer; EGL: External granular layer; I: Intermediate zone; IGL: Internal granular layer; M: Marginal layer; P: Purkinje cells; V: Ventricular side.Elements redrawn with permission from [Citation19,Citation20].](/cms/asset/bcf5cfc3-345d-4794-b4ff-b6157b53078c/iepi_a_12325151_f0001.jpg)
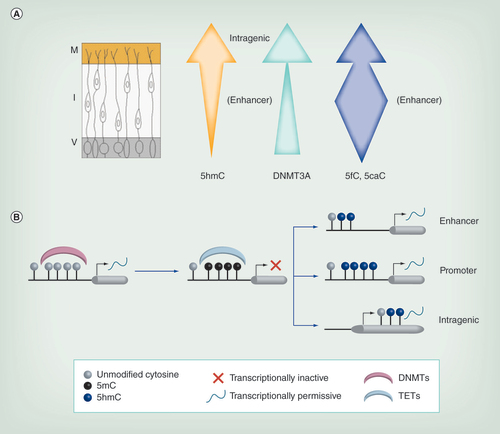
(A) As neurons migrate from the V, levels of 5hmC generally increase. Additionally its localization changes, with enrichment at intragenic regions in the M but at enhancers in the I. DNMT3A expression (green arrow) also increases across the neural tube. Levels of 5fC/5caC vary during migration, being more highly enriched in the enhancer regions particularly within the intermediate zone (blue arrow). (B) Methylation of naive DNA via DNMTs is often associated with transcriptional repression. This methylation can be reversed by TETs, which generate 5hmC, usually associated with transcriptional activation. Hydroxymethylation at the enhancer and promoter appears to be more transient and likely reflects active demethylation in these regions, while intragenic 5hmC is more stable and may be continually required for optimal transcription rates.
5hmC: 5-hydroxymethylcytosine; DNMT: DNA methyltransferase; I: Intermediate zone; M: Marginal zone; TET: Ten eleven translocase; V: Ventricular zone.
Magnetoencephalography scanning of boy aged 8 years shows user-friendly, non-invasive system with corresponding brain activity measurement on screen.