Figures & data
Table 1. Status of SMN-dependent drugs in clinical trials for spinal muscular atrophy.
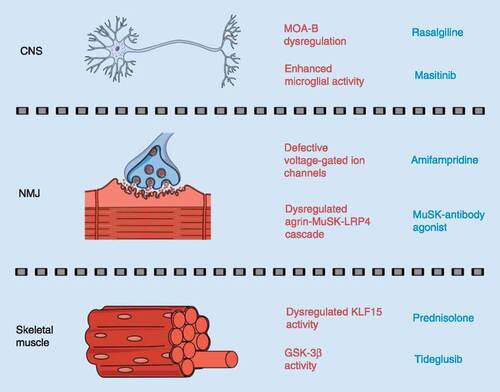
Overview of pathological molecular effectors or biological pathways (in red) that could be therapeutically modulated by commercially available drugs (in blue) to treat CNS, NMJ and skeletal muscle pathologies in spinal muscular atrophy.
NMJ: Neuromuscular junction.
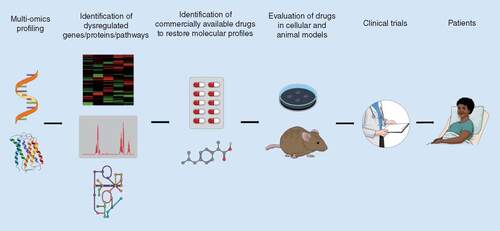
Proteomic and transcriptomic approaches can be used to define the differential expression patterns of genes, proteins and biological pathways between diseased and healthy states. Published literature or integrated publicly available drug databases allow for the uncovering of commercially available drugs that can restore the aberrant molecular profiles in the diseased state. The drugs then require to first be validated for safety and efficacy in relevant in vitro and in vivo models. Successful drug candidates can then be evaluated in clinical trials, with Phase I usually bypassed as information regarding safety and dosages are known. Finally, the drug is approved for patient use if it demonstrates benefits in clinical trials.