Figures & data
Table 1. Mean pharmacokinetic parameters of peginesatide in the plasma of male Sprague–Dawley rats after IV administration.
Figure 2. The plasma concentration–time profiles of peginesatide in male Sprague–Dawley rats after a single IV administration of peginesatide at a dose of 0.1, 0.5, or 5 mg·kg−1. Plasma concentration of peginesatide was determined by ELISA. Each data point represents the mean ± SD for three rats.
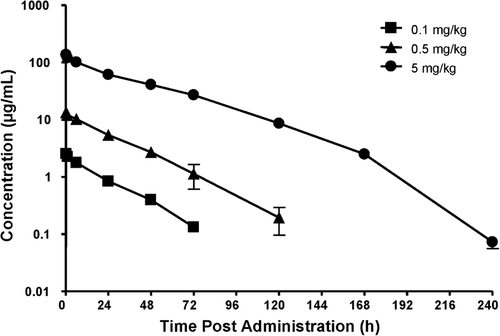
Table 2. Concentrations of the radioactivity in the tissues of albino rats after a single IV administration of 5 mg·kg−1 [14C]-peginesatide.
Figure 3. Log plasma concentration of the peginesatide-derived radioactivity in the plasma, eyes and skin of male albino (Sprague–Dawley) and pigmented (Long Evans) rats as a function of time after IV administration of 5 mg·kg−1 [14C]-peginesatide. Each value represents the mean ± SD for three animals.
![Figure 3. Log plasma concentration of the peginesatide-derived radioactivity in the plasma, eyes and skin of male albino (Sprague–Dawley) and pigmented (Long Evans) rats as a function of time after IV administration of 5 mg·kg−1 [14C]-peginesatide. Each value represents the mean ± SD for three animals.](/cms/asset/d110c973-46fb-49de-95d8-9d1e5f0b27c2/ixen_a_649310_f0003_b.gif)
Figure 4. Whole-body sections and subsequent autoradiographs of male Sprague–Dawley rats 72 h, 2 and 4 weeks following IV administration of 17 mg·kg−1 [14C]-peginesatide. Dark areas represent high radioactive concentrations.
![Figure 4. Whole-body sections and subsequent autoradiographs of male Sprague–Dawley rats 72 h, 2 and 4 weeks following IV administration of 17 mg·kg−1 [14C]-peginesatide. Dark areas represent high radioactive concentrations.](/cms/asset/2d8e89e6-1b22-4a68-add5-8fd602bea6ed/ixen_a_649310_f0004_b.gif)
Figure 5. Biolocalization of peginesatide to hematopoietic and EMH sites compared to muscle following a single IV administration of 17 mg·kg−1 [14C]-peginesatide. Each data point represents the mean ± SD for three rats.
![Figure 5. Biolocalization of peginesatide to hematopoietic and EMH sites compared to muscle following a single IV administration of 17 mg·kg−1 [14C]-peginesatide. Each data point represents the mean ± SD for three rats.](/cms/asset/57c1f231-f3fa-46a0-a2da-512bdf9c8ded/ixen_a_649310_f0005_b.gif)
Figure 6. Cumulative recovery of peginesatide-related radioactivity in urine and feces of male rats following IV administration of 5 mg·kg−1 [14C]-peginesatide. At 2 weeks after dosing, the remaining approximately 50% of the radioactivity not excreted was detected in the carcass. Each value represents the mean ± SD for three animals.
![Figure 6. Cumulative recovery of peginesatide-related radioactivity in urine and feces of male rats following IV administration of 5 mg·kg−1 [14C]-peginesatide. At 2 weeks after dosing, the remaining approximately 50% of the radioactivity not excreted was detected in the carcass. Each value represents the mean ± SD for three animals.](/cms/asset/c8061f36-c3bb-4b8e-aede-e8d9389b3d68/ixen_a_649310_f0006_b.gif)
Table 3. Pharmacokinetic parameters of peginesatide and associated radioactivity in plasma after single IV administration of [14C]-peginesatide at a dose of 5 mg·kg−1 to rats.
Table 4. Percent dose excretion of [14C]-peginesatide and associated radioactivity in rat urine and feces after single IV administration of 5 mg·kg−1.
Table 5. Day 14 concentrations of peginesatide and associated radioactive compounds in rat plasma and tissues after a single IV administration of 5 mg/kg [14C]-peginesatide.
![Figure 1. Chemical structure and sites of radiolabel for [14C]-peginesatide.](/cms/asset/9e58bd9d-4fd3-4f39-9f34-fd7658c97ec2/ixen_a_649310_f0001_b.gif)