Figures & data
Figure 1. Cytoophidia exist in various organisms. CTPS has been found forming filamentous structures in fruit flies (Liu, Citation2010), bacteria (Ingerson-Mahar et al., Citation2010), budding yeast (Noree et al., Citation2010), fission yeast (Zhang et al., Citation2014) and human cells (Carcamo et al., Citation2011; Chen et al., Citation2011). (A) In the fruit fly (Drosophila melanogaster) follicle cells, overexpressing CTPS-GFP (green) leads to long cytoophidia. (B) Cytoophidia, labeled by CTPS-GFP (green), is detectable in budding yeast (Saccharomyces cerevisiae). (C) Cytoophidia, labeled by CTPS-GFP (green), is detectable in fission yeast (Schizosaccharomyces pombe). (D) CTPS1-GFP (green) and IMPDH (red) form filamentous cytoophidia in human (Homo sapiens) HEK293T cells. Image in D is kindly provided by Chia Chun Chang and Li-Ying Sung from National Taiwan University. Nuclei are labeled by DNA dyes (magenta in A–C; blue in D). Scale bars, 10 μm. (see colour version of this figure at www.informahealthcare.com/bmg)
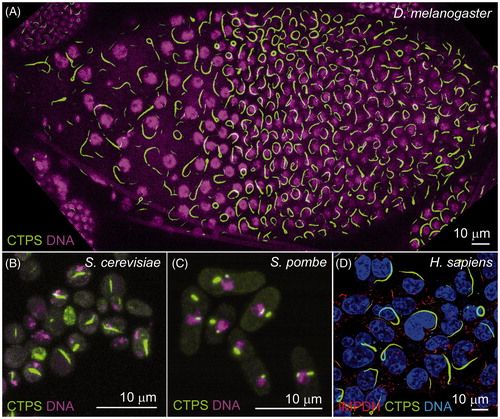
Figure 2. Schematic representation of proposed mechanisms of CTPS polymer assembly. (A) Mechanism demonstrated by Barry et al. (Citation2014). Active CTPS tetramers (left) undergo conformational change dependant on CTP concentration leading to polymer formation of interdigitated tetramer subunits. Multiple polymers associate into cytoplasmic filaments (right). (B) Mechanism proposed by Aughey et al. (Citation2014) and Noree et al. (Citation2014). Polymerization is dependent on dimerization/tetramerization state of CTPS. Catalytically active tetramers (left) dissociate into constituent dimers for inclusion into inactive cytoplasmic filaments (right). Both mechanisms rely on increasing CTP concentration to promote filament assembly (increasing left to right). (see colour version of this figure at www.informahealthcare.com/bmg)
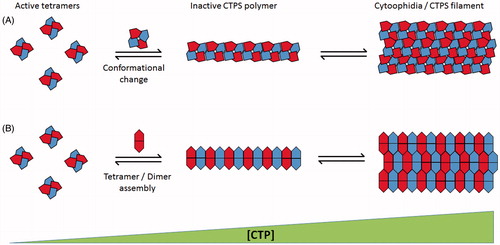
Figure 3. Schematic demonstrating hypothesized cellular functions of cytoophidia beyond regulation of enzymatic activity. (A) “Storage depot” downregulation or “Activator” upregulation of enzyme activity by filament assembly, as demonstrated by (Aughey et al., Citation2014; Barry et al., Citation2014; Noree et al., Citation2014) and (Chang et al., Citation2015; Strochlic et al., Citation2014), respectively. (B) Filament formation mediates structural roles analogous to cytoskeletal filaments as demonstrated in C. crescentus (Ingerson-Mahar et al., Citation2010). (C) The cytoophidium provides an intracellular scaffold for the sequestration of further cytoplasmic proteins. (D) Formation of intracellular filaments regulates traffic of CTPS between cellular compartments. (see colour version of this figure at www.informahealthcare.com/bmg)
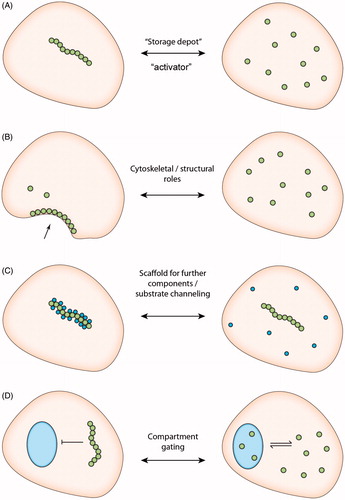
Table 1. Filament-forming proteins and their regulatorsa.
