Figures & data
Figure 1. Expression and location of ERβ-GFP fusion protein in MCF-7 cells in vitro under fluorescence microscope. (A) reflected light micrograph of MCF-7-GFP Cells. (B) corresponding fluorescence micrograph of MCF-7-GFP Cells. (C) reflected light micrograph of MCF-7-ERβ Cells. (D) corresponding fluorescence micrograph of MCF-7-ERβ Cells. The C terminus of the GFP was fused to the N terminus of the human ERβ. ERβ-GFP fusion protein stably expressed in ERβ-transfected MCF-7 cells localized both in the cytoplasm and the nucleus, with stronger GFP signal in the nucleus. (Original magnification ×400). (E) relatively numerical values of mean fluorescence intensity in nucleus and in cytoplasm in ERβ-transfected MCF-7 cells compared with that of background. Data were given as mean ± SD. **p < 0.01 versus corresponding mean fluorescence intensity in cytoplasm.
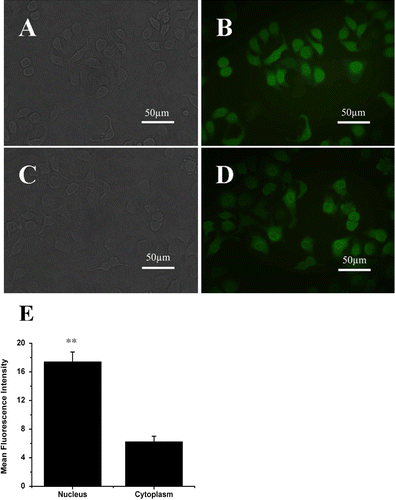
Figure 2. Expression of ERβ and cathepsin D mRNA. (A) MCF-7 cells were stably transfected with pEGFP-C3 or pEGFP-hERβ. After 24 h of treatment with 10−9 M E2 (+E2) or control vehicle ethanol (−E2), ERβ and CTSD expressions were detected by RT-PCR with β-actin as the reference gene. The PCR products have a size of 393 and 377 bp for ERβ and CTSD, respectively. (Marker. DNA Marker-B; wild type. Nontransfected MCF-7 cells; GFP. Cells stably transfected with pEGFP-C3; ERβ. Cells stably transfected with pEGFP-hERβ). (B) Semiquantitative calculation of relative mRNA expression of ERβ normalized to β-Actin levels. (C) Semiquantitative calculation of relative mRNA expression of CTSD normalized to β-Actin levels. Each experiment was performed three times independently and results were expressed as means ± SD. ** p ≤ 0.01, ***p ≤ 0.001 versus corresponding wild type.
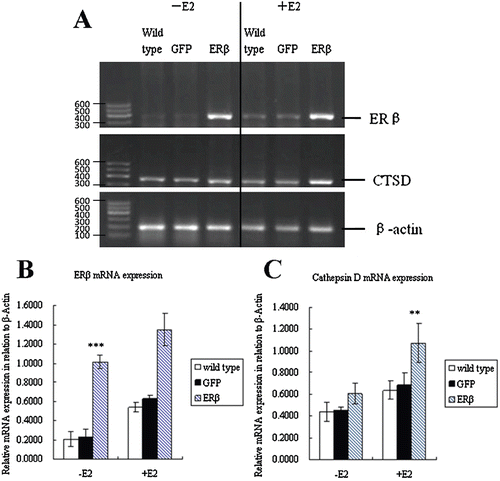
Figure 3. Cell growth of MCF-7 cells stably expressing GFP or ERβ. MCF-7 cells were stably transfected with pEGFP-C3 or pEGFP-hERβ. Wild type and transfected MCF-7 cells overexpressing GFP or ERβ were cultured up to 5 days in serum-free medium in the presence of 10−9 M E2 (+E2) or control vehicle ethanol (−E2). Relative viable cell numbers were determined by MTT assay on day 0, 2, 3, 4, and 5. Viable cell numbers are expressed as percentage relative to day 0. Each experiment was performed three times independently and results were expressed as means ± SD. *p ≤ 0.05, ***p ≤ 0.001 versus corresponding wild type.
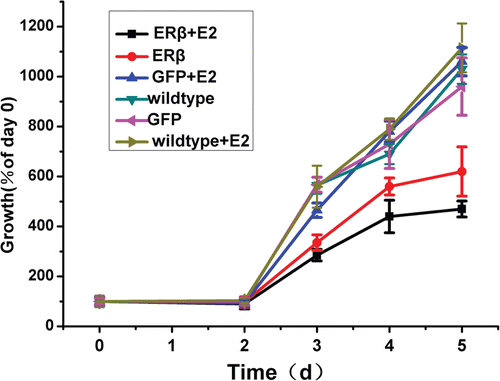
Figure 4. ERβ enhances the antiproliferative effects of genistein, raloxifene, and TSA to inhibit breast cell proliferation. (A) genistein inhibits proliferation of MCF-7 cells to a greater extent when ERβ is expressed. MCF-7-ERβ, MCF-7-GFP, and wild-type MCF-7 cells were treated with increasing concentrations of genistein for 72 h and the relative viable cell numbers were determined by MTT assay. Each experiment was performed three times independently and results were expressed as means ± SD. Similar experiments were conducted on MCF-7-ERβ, MCF-7-GFP, and wild type MCF-7 cells treated with raloxifene (B) and TSA (C). Each experiment was performed three times independently and results were expressed as means ± SD: **p ≤ 0.05, **p ≤ 0.01 versus IC50 of corresponding agent in MCF-7 wild-type cells; #p ≤ 0.05, versus IC50 of corresponding agent in MCF-7-GFP Cells.
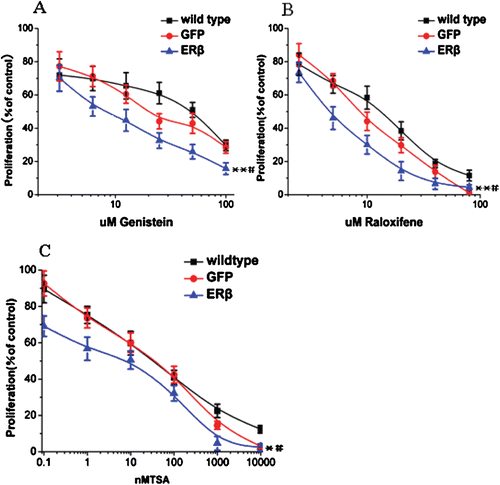
Figure 5. Motility of MCF-7 cells stably expressing GFP or ERβ. MCF-7 cells were stably transfected with pEGFP-C3 or pEGFP-hERβ. Wild-type and transfected MCF-7 cells overexpressing GFP or ERβ were cultured in serum-free medium in the presence of 10−9 M E2 (+E2) or control vehicle ethanol (−E2). After 24 h of treatment, wound-induced migration was triggered by scraping the cells with a blue tip, and the scratch was imaged immediately (0 h). The cells were imaged again at 48 h and 72 h after the scratch. Each experiment was performed three times independently. (A) images of a representative assay at 0, 48, and 72 h are shown here. (B) The percent of wound filling was calculated by measuring the remaining gap on the images. The ratio of the gap space at 48 h and 72 h over the gap space at 0 h gives the percentage of wound filling. Results were obtained from three separate experiments and are expressed as means ± SD. ***p ≤ 0.001 versus corresponding wild type.
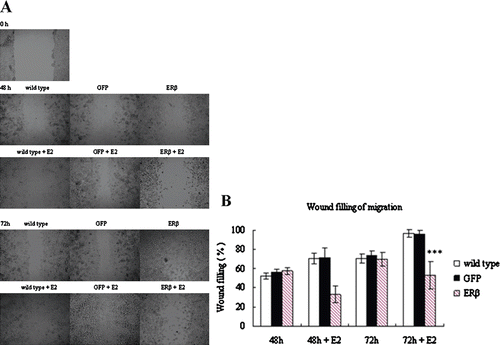
Figure 6. ERβ inhibits tumor formation and growth of MCF-7 cells in mice xenografts. 3 × 106 MCF-7-ERβ and MCF-7-GFP monoclonal cells suspended in 200 μL normal medium were transplanted subcutaneously into the right and left armpit, respectively, of six female BALB/c-nu nude mice without Matrigel. Of the six mice implanted with MCF-7-ERβ and MCF-7-GFP, MCF-7-GFP inoculation resulted in the development of 6/6 tumors, whereas MCF-7-ERβ inoculation resulted in only 2/6 tumors developing. (A) Representative image of the developed xenografts at day 15. The arrow points to the site of grafting of MCF-7 cells stably transfected with GFP or ERβ. (B) Tumor growth of the developed grafting of MCF-7 cells stably transfected with GFP or ERβ. Tumor diameters were measured twice weekly and tumor volumes were calculated according to equation (width2 × length)/2. *p ≤ 0.05, ***p ≤ 0.001 versus corresponding xenografts grafting of MCF-7 cells stably transfected with ERβ.
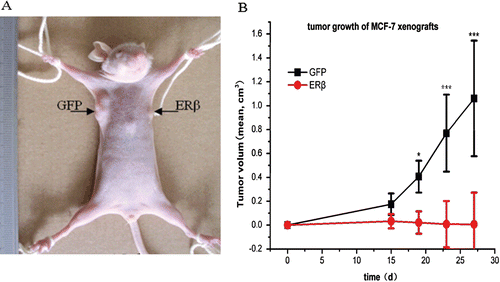
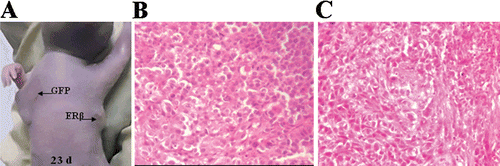
Figure 7. Representative image and pathological examination of xenografts of polyclonal MCF-7 cells stably transfected with GFP or ERβ. 2 × 106 ERβ-transfected and GFP-transfected polyclonal MCF-7 cells suspended in 200 μl normal medium were transplanted subcutaneously into the right and left armpit, respectively, of six female BALB/c-nu nude mice without Matrigel. (A) Representative image of xenografts at day 23. (B) Representative image of pathological examination of MCF-7-GFP xenografts by H&E stain (original magnification ×100). (C) Representative image of pathological examination of MCF-7-ERβ xenografts by H&E stain (original magnification ×100).