Figures & data
Figure 1. Experimental protocols and animal groups. (A) 96 rats were divided into 2 groups (n = 48 per group): the control group (sham) and the ischemic preconditioning group (IPC). In each group, the hearts were harvested at successive time points (days 0, 1, 2, 3, 5 and 7) after myocardial IPC or sham operation (n = 8 per time point). Sham hearts were operated without ligating the coronary artery. (B) Rats were divided into 4 groups (n = 8 per group) and injected with the CD25 monoclonal antibody, NDS61, or control IgG, 24 h before 30-min ischemia (30 min I) or the second sham surgery. The hearts were harvested after a 48-h reperfusion (48 h R).
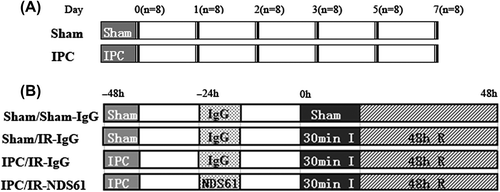
Figure 2. Myocardial ischemic preconditioning (IPC) caused the protein expression of FoxP3. At 0, 1, 2, 3, 5, and 7 days after myocardial IPC or sham (non-preconditioned) surgery, rat hearts were harvested and quantitatively analyzed for FoxP3 expression, using the Western blotting method. The FoxP3 protein levels are represented as a percentage of their respective sham control. Data are presented as the mean ± S.D.; ***p < 0.001, compared to nonpreconditioned rats, n = 8 per group.
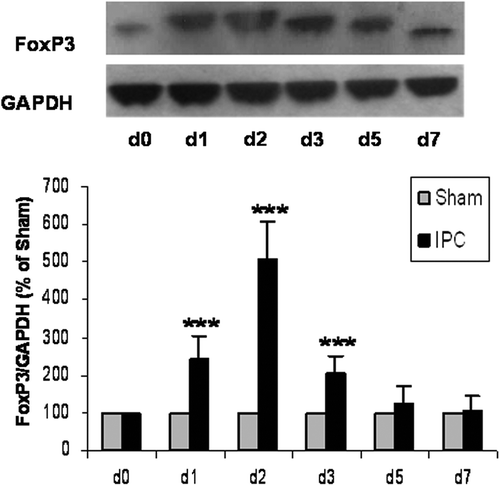
Figure 3. NDS61 administration significantly reduced Tregs in preconditioned rats. Preconditioned rats were injected with NDS61 or control IgG on day-1, before 30-min myocardial ischemia. Just before 30-min myocardial ischemia, FoxP3 expression in the Spleen (A) and heart (B) were measured by Western blotting analysis. Data are presented as the mean ± S.D.; ***p < 0.001 compared to IPC/IR-IgG. IPC, ischemic preconditioning; IR, ischemia-reperfusion injury; Treg, regulatory T.
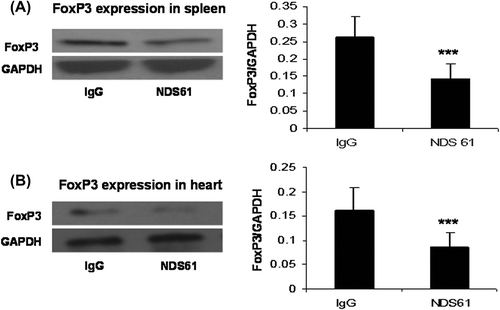
Figure 4. Treg cell depletion inhibits anti-inflammatory effect of IPC. On day-1, before 30-min myocardial ischemia, preconditioned rats were injected with NDS61 or control IgG. At the end of 48-h reperfusion, some heart sections were stained with hematoxylin and eosin to assess total inflammatory cell infiltration in the different treatment groups. Data are presented as the mean ± S.D.; ***p < 0.001 compared to Sham/IR-IgG; †p < 0.05 compared to IPC/IR-IgG. IPC, ischemic preconditioning; IR, ischemia-reperfusion (original magnification 200×).
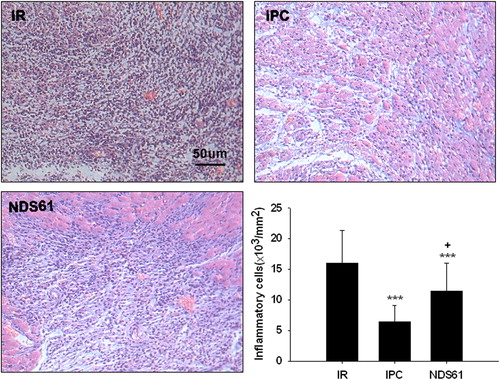
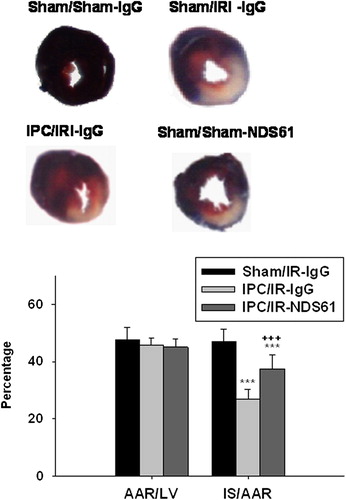
Figure 5. Treg cell depletion increases myocardial infarct size. On day-1, before 30-min myocardial ischemia, preconditioned rats were injected with NDS61 or control IgG. At the end of the 48-h reperfusion, the infarct size was determined by TTC staining (n = 8 per group). Values are expressed as mean ± S.D. ***p < 0.001 compared to Sham/IR-IgG; †††p < 0.001 compared to IPC/IR-IgG. LV, left ventricle; AAR, area at risk; IS, infarct size; IPC, ischemic preconditioning; IR, ischemia-reperfusion injury.