Figures & data
Table 1. Structures and synthetic data of hydrazide and hydrazine derivatives.
Table 2. FlexX docking scores and human cathepsin D and P. falciparum Plasmepsin-II inhibition data of hydrazide and hydrazine derivatives.
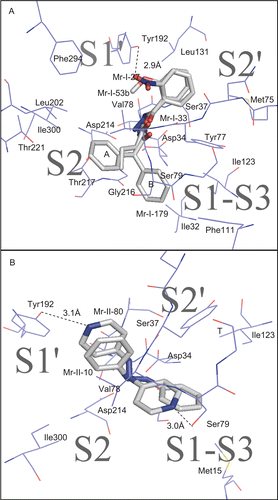
Figure 1. Enzyme inhibition plots as a function of hydrazide and hydrazine compounds concentrations.
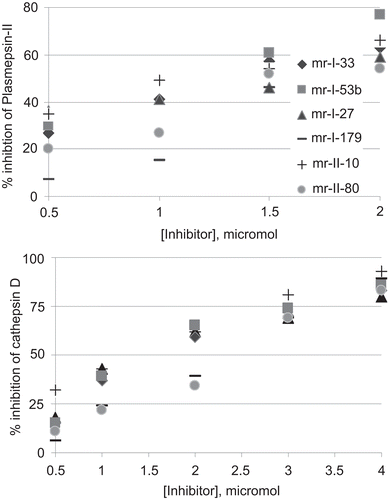
Figure 2. Interactions of (A) hydrazide and (B) hydrazine transition state isosteres with catalytic aspartates of plasmepsin-II. Electrostatic interactions of hydrazide and hydrazine moieties of inhibitors with Asp34 and Asp214 residues are shown by broken lines (distances in Angstrom).
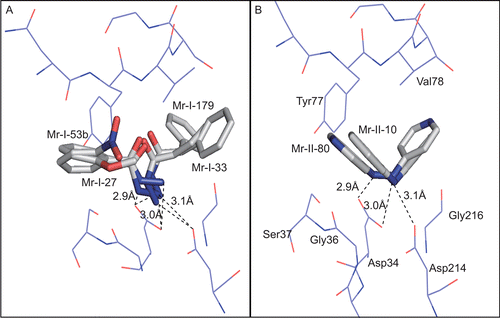
Figure 3. Docking of (A) hydrazides and (B) hydrazine compounds in the substrate-binding cleft of Plasmepsin-II. Interactions of different enzyme sub-sites with inhibitors are shown. Hydrogen bonds are indicated as broken lines (distances in Angstrom). The two phenyl rings of Mr-I-179 are denoted as ‘A’ and ‘B’.