Figures & data
Figure 2. (A) The molecular structure of tetrabromide 9 showing the atom numbering scheme. Thermal ellipsoids are drawn at the 40% probability level. (B) packing diagram and H bonding geometry along the a-axis. (symmetry code a: 2-x. 1-y.1-z).
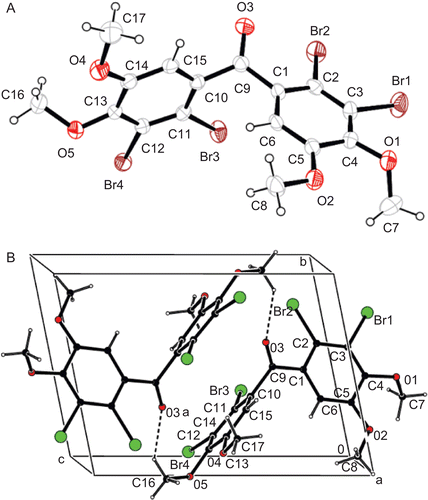
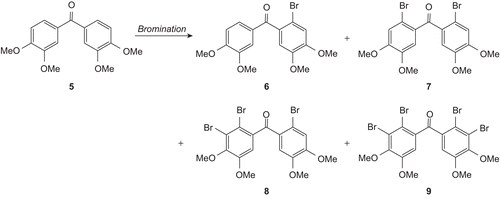
Table I. Bromination reactions of the compound 5 (1.0 eq.) at different conditions and % yield of the product(s).
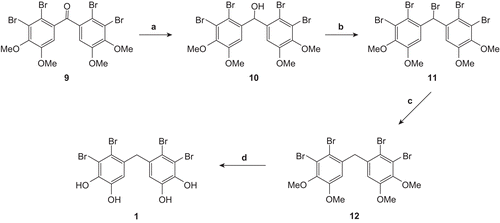
Scheme 2. Reagents and conditions: (a) NaBH4/THF-MeOH. 0°C -RT. 12 h; then dilute HCl. 93%; (b) PBr3/NEt3. benzene. 0°C -RT. 12 h. 93%; (c) n-Bu3SnH. AIBN/THF. reflux.77%; (d) BBr3/CH2Cl2. 0°C -RT. 24 h; then MeOH. 97%.
Scheme 3. Reagents and conditions: (a) KOH-NH2NH2/(OHCH2)2. 110-190°C. 6 h. 86%; (b) BBr3. CH2Cl2.96%.
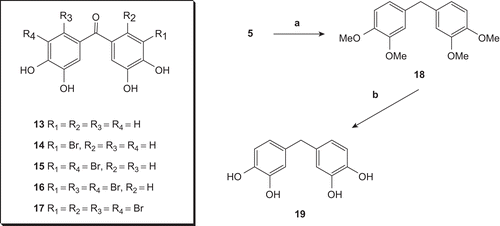
Table II. Comparison of ferric ions (Fe3+) reducing ability by Fe3+-Fe2+ transformation methods, Cu+ reducing ability by CUPRAC method, ferrous ion (Fe2+) chelating activity of bromophenol derivatives (1, 13–17 and 19) and standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox at the same concentration (10 μg/mL).
Table III. Concentration required for 50% scavenging (IC50) of DPPH·. ABTS·+. DMPD·+ and O2·- radical scavenging activities of bromophenol derivatives (1. 13–17 and 19) and standard antioxidant compounds such as BHA. BHT. α-tocopherol and trolox (DPPH·: 1.1-diphenyl-2-picryl-hydrazyl free radical. ABTS·+: 2.2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid. DMPD·+: N.N-dimethyl-p-phenylenediamine dihydrochloride radical. O2·-: superoxide anion radicals.