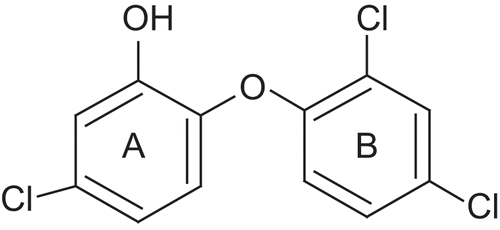
Figures & data
Scheme 1. Synthesis of the oxadiazole thiones, triazoles, and pyrazoline derivatives of diphenyl ethers.
Ar, ortho-, meta-, or para- phenoxy phenyl (a, b, c) in case of oxadiazolethione (5a, 5b, and 5c) and triazole derivatives (7a, 7b, and 7c). Ar, meta-phenoxy phenyl for (9).
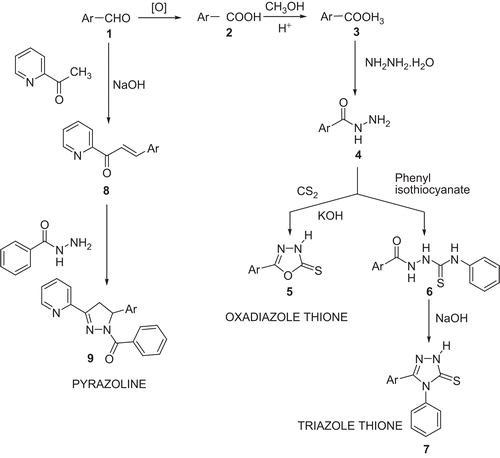
Table 1. Structures of the compounds synthesised and some of their physical properties.
Scheme 2. Synthesis of isoxazoles
Ar, meta-phenoxy phenyl; R = H (10a), 4-chloro (10b), and 4-methoxy (10c).
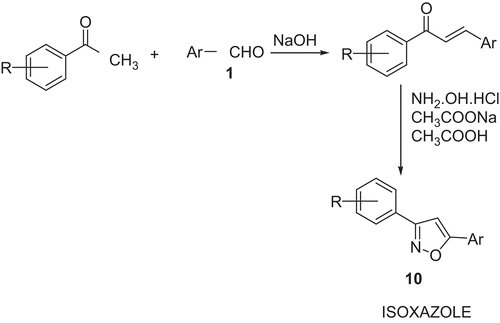
Table 2. Biological activity of the diphenyl ether derivatives against H37Rv strain of M. tuberculosis and inhibitory activity against ENR.