Figures & data
Figure 1. Synthesis of 5-(5-nitroaryl)-2-substituted-1,3,4-thiadiazoles. Reagents and conditions; (a) NH4Fe(SO4)2, H2O, reflux; (b) NaNO2, HCl, Cu; (c) Thiourea, EtOH, reflux, then HCl; (d) R-X, ethanol, KOH, rt.
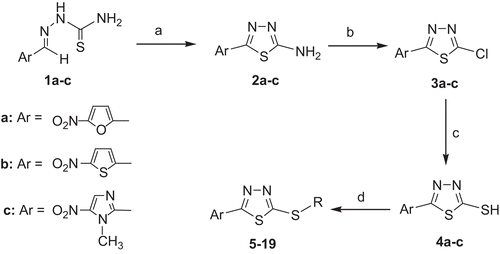
Table 1. Structures and in vitro activities of compounds 5–19 against the promastigote form of L. major.