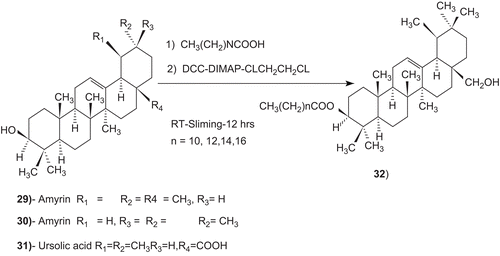
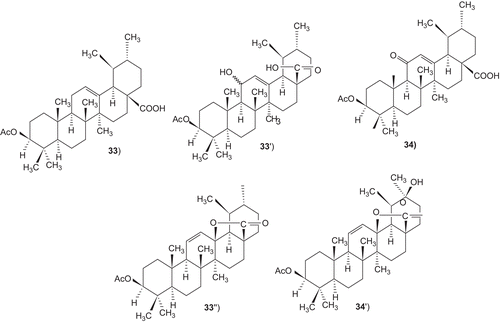
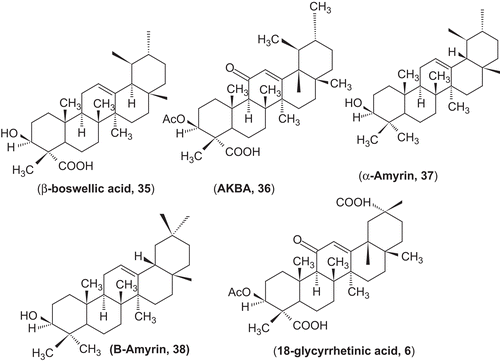
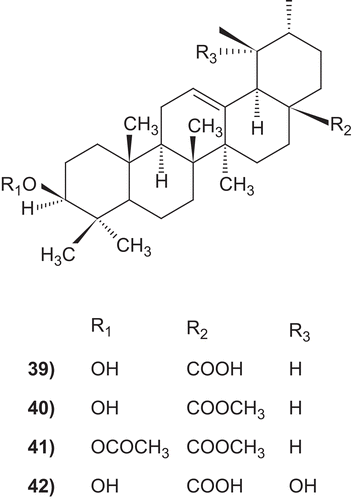
Figures & data
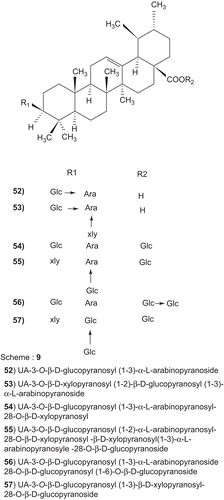
Scheme 9. (52) UA-3-O-β-d-glucopyranosyl (1-3)α-l-arabinopyranoside (53) UA-3-O-β-d-xylopyranosyl (1-2)-β-d-glucopyranosyl (1-3)α-l-arabinopyranoside (54) UA-3-O-β-d-glucopyranosyl (1-3)α-l-arabinopyranosyl-28-O-β-d-xylopyranosyl (55) UA-3-O-β-d-glucopyranosyl (1-2)α-l-arabinopyranosyl-28-O-β-d-xylopyranosyl -β-d-xylopyranosyl(1-3)α-Larabinopyranosyle-28-O-β-d-glucopyranoside (56) UA-3-O-β-d-glucopyranosyl (1-3)α-l-arabinopyranoside 28-O-β-d-glucopyranosyl (1-6)-O-β-d-glucopyranoside (57) UA-3-O-β-d-glucopyranosyl (1-3)β-d-xylopyranosyl-28-O-β-d-glucopyranoside.