Figures & data
Scheme 1. Reagent and condition: (i) Br2, AcOH, rt, 1h; 95% (ii) NaOH, Cu, H2O, reflux, 18h; 60% (iii) CH2Br2,CuO, K2CO3, DMF, 140°C, 4h; 70% (iv) MeOH, diamine, rt, 4h (v) NaBH4, rt, 4h, 80–90%.
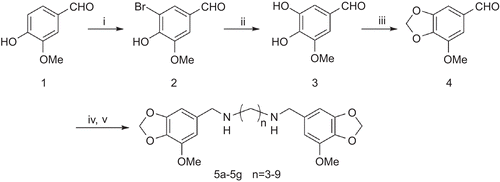
Table 1. Inhibition activity of AChE and BuChE.
Figure 2. Compounds 5a–5g Aβ1-42 fibril inhibition compared with that of curcumin. The test was conducted in the presence of 20 μM compounds.
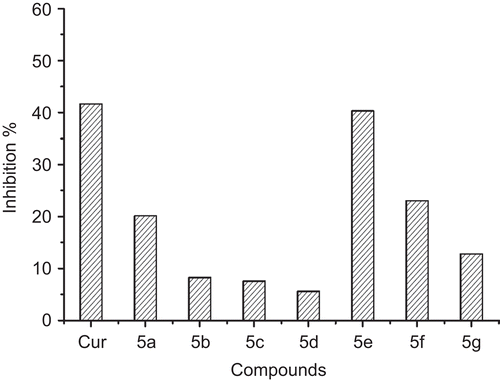
Table 2. MTT assay of SH-SY5Y cell viability.