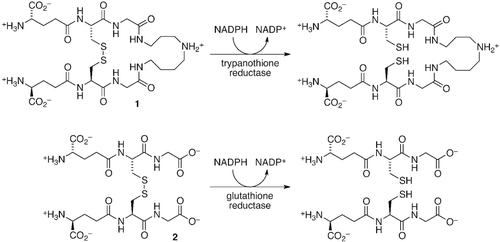
Figures & data
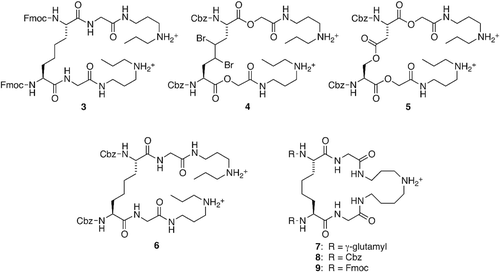
Table 1. Inhibition of TR by compounds 3–5 and by previously reported inhibitors 6–9a
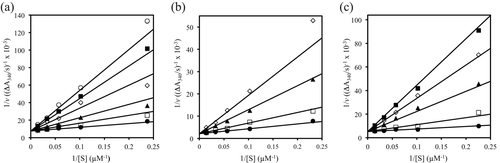
Figure 4. Competitive inhibition of TR y 3–5. (a) Lineweaver–Burk (L–B) plot for TR inhibition in the absence (•) and presence of 3 at 2.2 µM (□), 5.1 µM (▴), 10.2 µM (◊), 15.3 µM (▪), and 19.7 µM (○). (b) L–B plot for TR inhibition in the absence (•) and presence of 4 at 18.7 µM (□), 62.5 µM (▴), and 109.3 µM. (c) L–B plot for TR inhibition in the absence (•) and presence of 5 at 15.3 µM (□), 61.1 µM (▴), 107.0 µM (◊), and 152.9 µM (▪). Each point represents the average of two to four experimental determinations; the lines are based on theoretical values derived from the nonlinear least-squares fit of the data sets using the COMP program Citation31.