Figures & data
Table 1. Inhibition data of chlorthalidone 1, indapamide 2 and furosemide 3 against isoforms hCA I, II, IX and XII.
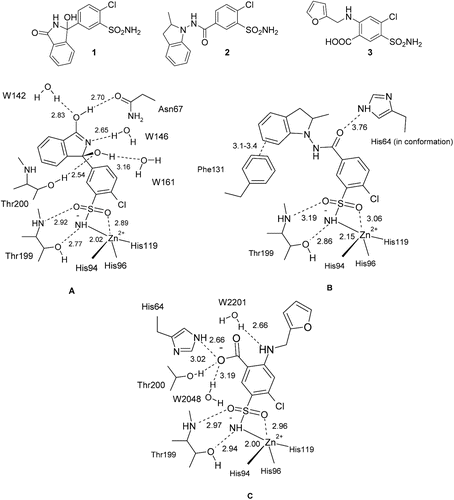
Figure 1. Comparison of interactions in which the thiazide/high-celling diuretics chlorthalidone 1 (A), indapamide 2 (B) and furosemide 3 (C) participate when bound to the hCA II active site, as observed in the corresponding X-ray crystal structures (PDB files 3F4X, 3BL1 and 1Z9Y).
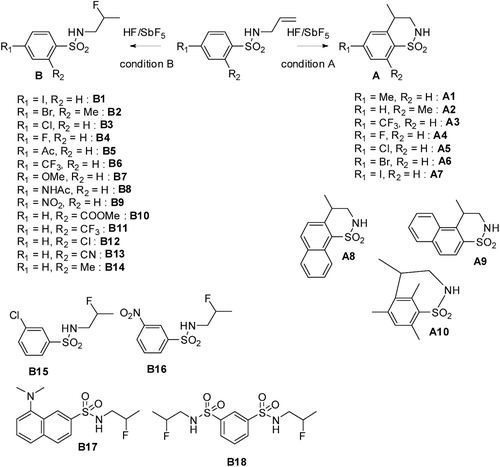
Scheme 1. Synthesis of benzofuzed sultams A and β-fluorinated benzenesulfonamides B in superacid HF/SbF5. Condition A: 10 min, −20°C, substrate concentration c = 0.16 mol·L−1, % mol SbF5 = 13.6; Condition B: 10 min, −20°C, substrate concentration c = 0.33 mol·L−1, % mol SbF5 = 3.8.
Scheme 2. Synthesis of 4-aminobenzofuzed sultams 4, 5, 6 and 9. (a) HF/SbF5 (% mol SbF5 = 3.8), −20°C, 10 min, 42%. (b) CH3I, K2CO3, DMF, rt, 12 h, >95% (c) CuI, L-proline, octylamine, K3PO4, DMSO, 90°C, 24 h, 62%. (d) HF/SbF5 (% mol SbF5 = 13.6), −20°C, 10 min, 62%.(e) CuI, L-proline, 2-aminobutanol, Cs2CO3, DMSO, 90°C, 24 h, 70%. (f) CuI, L-proline, aq. NH3, K3PO4, DMSO, 90°C, 24 h, 81%. (g) allylbromide, K2CO3, DMF, rt, 12 h, 83%. (h) CuI, L-proline, morpholine, K3PO4, DMSO, 90°C, 24 h, 70%. (i) HF/SbF5 (% mol SbF5 = 3.8), −20°C, 10 min, 69%.
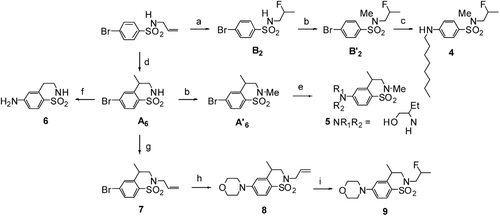
Table 2. hCA I and II inhibition data with sulfonamides A1–A10, by the CO2 hydrase methodCitation15.
Table 3. CA inhibition data with β-fluorinated derivatives B1–B18 and 4-aminobenzenesulfonamides 4–6, 9 and SA as standard inhibitor, against isozymes I, II, IX and XII, by a stopped-flow CO2 hydrase assay.