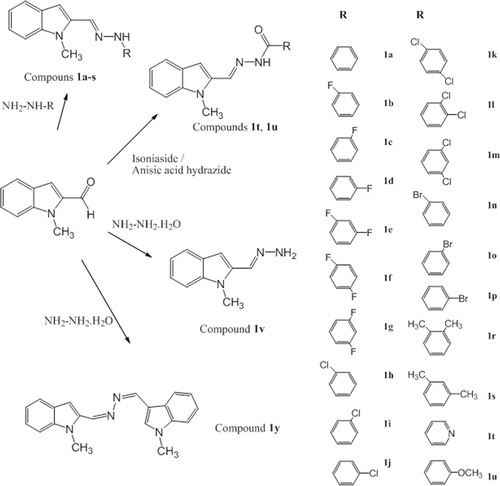
Figures & data
Figure 1. Parts of the MLT molecule modified to develop new indole-based MLT analogue compounds. MLT, melatonin.
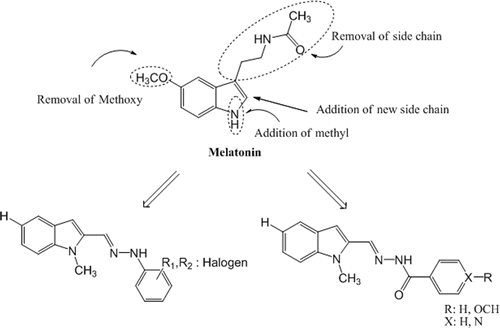
Figure 3. Oxidation of DCFH via reactive oxygen species in erythrocytes after incubation with ascorbic acid, MLT and newly synthesized analogs for 60 min. Control group represents cells incubated with PBS without any compound addition. Values are mean ± SD of three individual experiments. *p < 0.05, compared to control group. **p < 0.005, compared to control group. #p < 0.0005, compared to control group. MLT, melatonin.
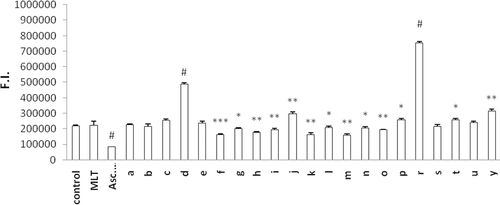
Figure 4. Antioxidant effect of indole-analogs on H2O2-induced oxidation of DCFH in erythrocytes. Control group represents cells incubated with PBS without any compound addition. Values are mean ± SD of three individual experiments. §p < 0.005, compared with control. *p < 0.05, compared with H2O2 group. **p < 0.005, compared with H2O2 group. #p < 0.0005, compared with H2O2 group.
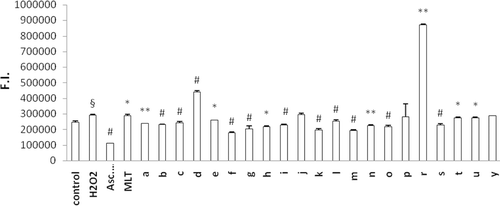
Figure 5. Effect of MLT analogs on LDH leakage from CHO cells into the culture media. Control group represents cells incubated in regular media without any compound addition. % 2.5 DMSO is used to cause membrane damage where ascorbic acid is used as an antioxidant. *p < 0.05, **p < 0.005 compared with control.
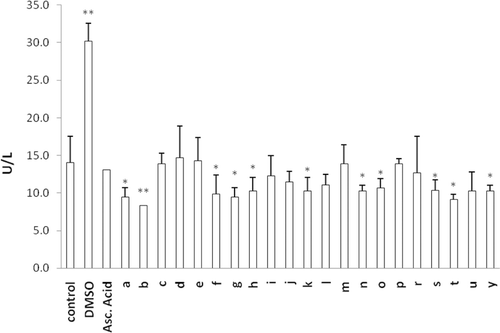
Figure 6. CV voltammograms of 20 µg/mL 1f and 10 µg/mL 1g, 1y, and 1u in 0.1 M H2SO4. (A,B,C, and D): (A) 20 µg/mL 1f, 40% MeOH (B) 10 µg/mL 1g, 50% MeOH(C) 10 µg/mL 1y 20% MeOH (D) 10 µg/mL 1u 30% MeOH.
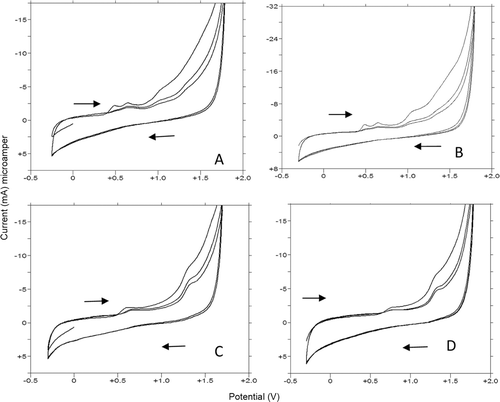
Figure 7. CV voltammograms of 1f, 1g, 1y, and 1u. (A,B,C, and D): (A) 20 µg/mL 1f, pH 6 FT (B) 10 µg/mL 1g, pH 4.7 AT (C) 10 µg/mL 1y, pH 7.0 BRT (D) 10 µg/mL 1u, pH 10 BRT.
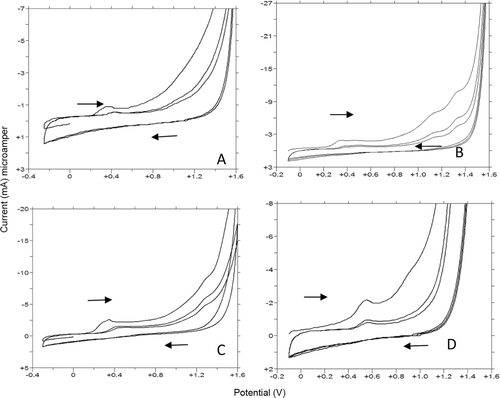
Table 1. The results of the scan rates experiments on 1f, 1g, 1y, and 1u.
Table 2. Calibration parameters of 1f, 1g, 1y, and 1u.