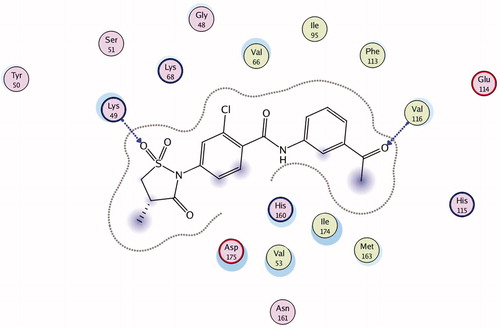
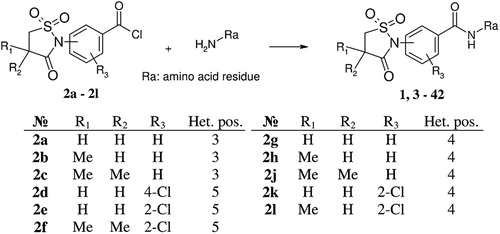
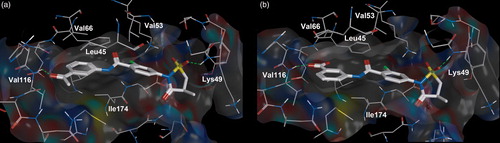
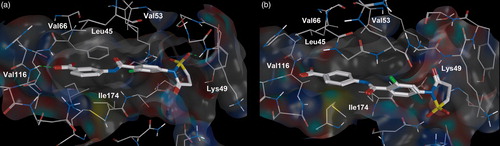
Figures & data
Table 1. Compounds 21–39 with para-position of the thiazolidine cycle relative to carboxylamide fragment and the data related to their in vitro screening.
Table 2. Compounds 40–42 containing the fragments of amino acids with aliphatic chains and the data related to their in vitro screening.
Table 3. Residual activity of protein kinases (%) with added inhibitor (10 μM) and ATP (100 μM).