Figures & data
Table 1. Physicochemical and analytical data of compounds 1–28.
Table 2. Physicochemical and analytical data of the scheme 2 compounds 29–51.
Table 3. 1H NMR data of compounds 1–51.
Table 4. Cytotoxic effects (LC50; μM)a of the active compounds on some human tumor cell lines using the MTT assay.
Table 5. Minimal inhibitory concentrations [MIC, µg/mL (μM)] of the tested compounds.
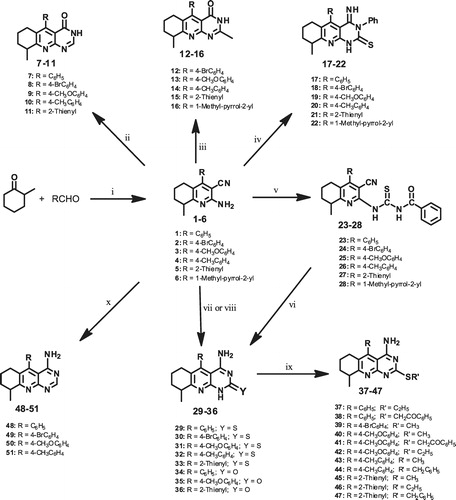
Scheme 1. Reagents and reaction conditions: (i) 2-methylcyclohexanone (0.01 mol), approp. aldehyde (0.01 mol) malononitrile (0.01 mol), NH4OAc (0.08 mol) in Abs. EtOH (50 mL), reflux 3–6 h; (ii) approp. starting compd. (0.01 mol), formic acid (5 mL), heated in boiling water bath 30 min; (iii) approp. starting compd. (0.01 mol), Ac2O (5 mL), conc. H2SO4 (0.5 mL), heated in boiling water bath 10 min; (iv) approp. starting compd. (0.01 mol), PhNCS (0.01 mol) in pyridine (15 mL), reflux 2 h; (v) approp. starting compd. (0.02 mol), PhCONCS (0.02 mol), dry acetone (10 mL), reflux 3 h; (vi) approp. thioureido deriv. (0.02 mol), 1N NaOH (5.5 mL), EtOH (10 mL), heated in boiling water bath 30 min; (vii) approp. starting compd. (0.001 mol), urea or thiourea (0.005 mol), fused at 260–300 °C, sand bath 1 h; (viii) approp. starting compd. (0.001 mol), urea or thiourea (0.005 mol), heated in microwave reactor at 250 °C, 15 min; (ix) approp. thione (0.02 mol), 1N NaOH (5 mL), EtOH (2 mL), alkyl or aralkyl halide (0.022 mol), stirred at r.t., 2–3 h and (x) approp. starting compd. (0.01 mol), HCONH2 (10 mL), reflux, 2–3 h.

![Figure 1. 1-Benzoyl-3-[3-cyano-8-methyl-4-(1-methyl-1H-pyrrol-2-yl)-5,6,7,8-tetrahydroquinolin-2-yl]thiourea.](/cms/asset/6ca507ba-b294-4853-94cd-4640502c2f5c/ienz_a_787421_f0002_b.jpg)