Figures & data
Figure 2. Phylogenetic tree of the α, β and γ CAs from selected eukaryotic and prokaryotic species. The tree was constructed using the program PhyML 3.0. Branch support values are reported at branch points. Organisms, accession numbers and cryptonyms of the sequences used for the phylogenetic analysis have been indicated in .
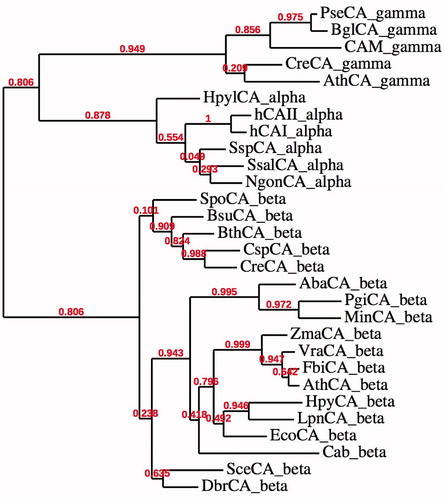
Table 1. CA class, organisms, accession numbers and cryptonyms of the sequences used in the phylogenetic analysis.
Figure 3. Sulfa drugs in clinical use (7–11) and prontosil 6, the lead molecule generating this class of pharmacological agents. These compounds are structurally similar to pABA 2 and compete with this compound for the biosynthesis of DHP 3Citation58. Many other analogs are knownCitation58.
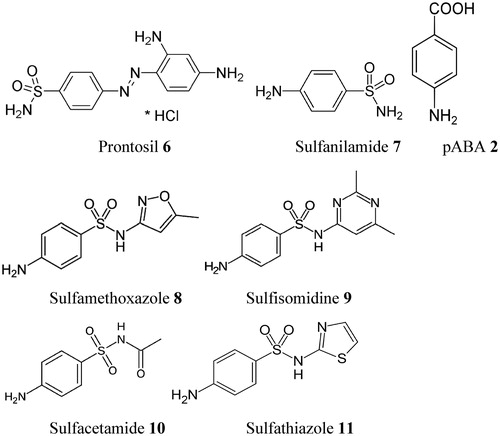
Figure 4. Covalent adduct of DHPP with the sulfa drug sulfathiazole, of type 14, as determined by X-ray crystallography (A) and the detailed interactions in which 14 participates when bound to the enzyme (B)Citation58.
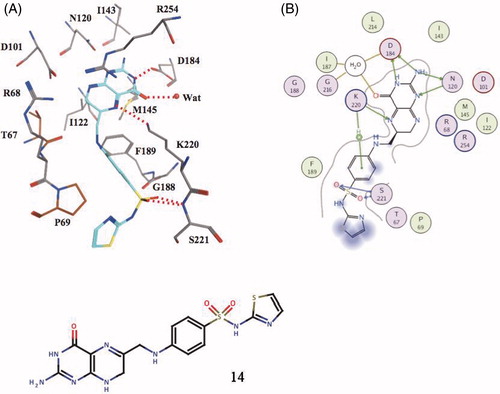
Figure 5. Chemical structure of the DHFR inhibitor trimethoprim 12. Its congeners (brodimoprim, tetroxoprim, etc.) have slightly different moieties replacing the 4-methoxy group of 12Citation57. The new DHFR inhibitor iclaprim 13 is also shownCitation57.
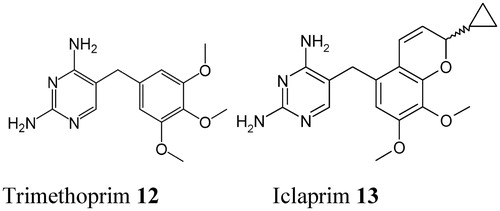
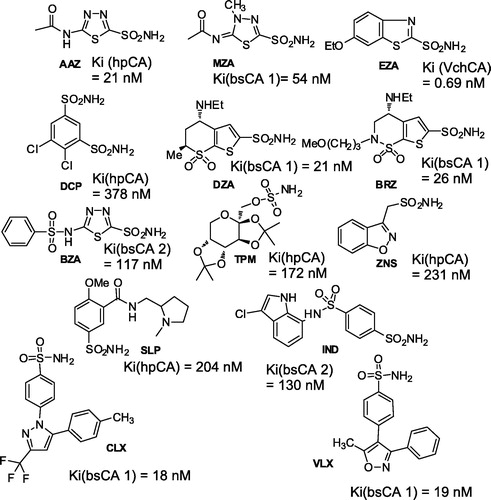
Figure 6. Inhibition data of hpCA (α isoform), bsCA 1, bsCA 2 and VchCA with sulfonamides/sulfamates in clinical useCitation12,Citation13,Citation59,Citation61.