Figures & data
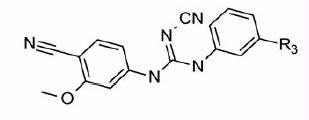
Figure 1. Chemical structures of select IMPDH inhibitors used for the pharmacophore model developmentCitation12.
Figure 2. The five-point pharmacophore model – AADHR – for IMPDH inhibitors with VX-148 (3, ) and the design strategy based on 3 (Parts A, B and C) utilized for systematic modifications. A: Acceptor (light pink); D: Donor (cyan); H: Hydrophobic (green) and R: Ring (orange) features.
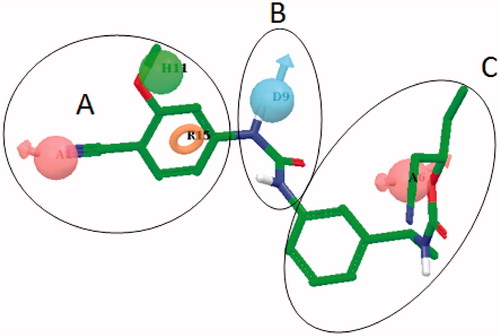
Table 1. In vitro inhibitory and cellular potencies of Part A modifications.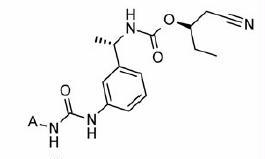
Scheme 1. Syntheses of compounds 13–27. Reagents and conditions: (a) NaBH4, EtOH, RT; (b) DPPA, DBU, toluene; (c) PPh3, THF:Water; (d) L-(+)-tartaric acid, MeOH; (e) NaOH, EtOAc; (f) H2, Pd(OH)2/C, EtOH; (g) KCN, DMSO; (h) 10, CDI, EtOAc, RT; (i) phenyl chloroformate, DCM, RT; (j) DIPEA, EtOAc, RT; (k) DCM, RT, 6 h.
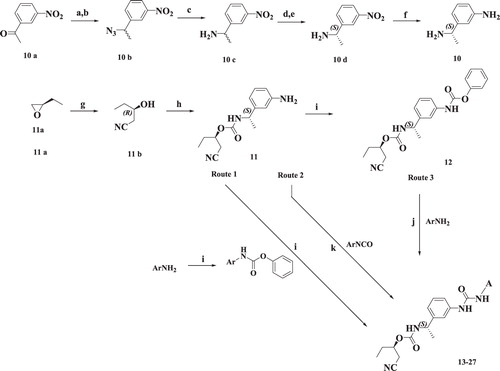
Scheme 2. Syntheses of compounds 30–35. Reagents and conditions: (a) DCM, RT; (b) (i) CuSO4:SiO2; (ii) methanolic NH3, THF; (c) MeI, K2CO3, acetone; (d) NaNHCN, 2-propanol, 80 °C, 1 h; (e)1,1′-thiocarbonyldi-2(1H)-pyridone, DCM, RT; (f) (i) HCl:1,4-dioxane, RT; (ii) 11b, CDI, EtOAc, RT; (g) MeI, DMF, RT; (h) EtOH, reflux.
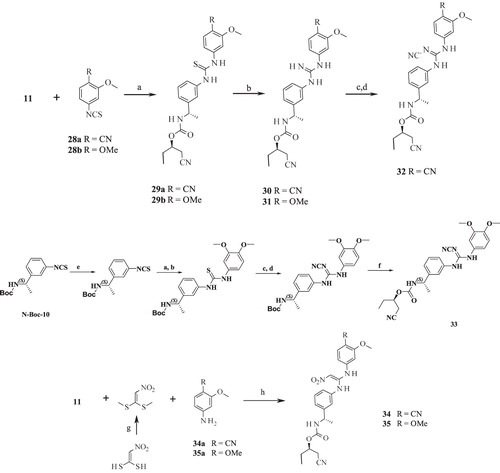
Scheme 3. Syntheses of compounds 36–39. Reagents and conditions: (a) KI (cat.), AcCN, RT; (b) HATU, DIPEA, THF, RT.
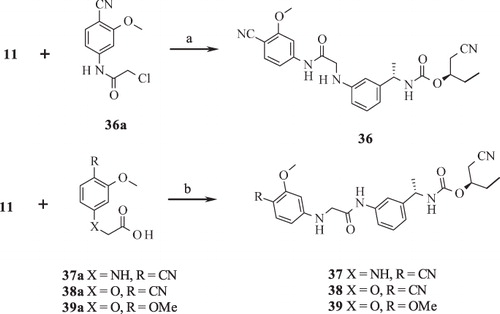
Scheme 4. Syntheses of compounds 40–44. Reagents and conditions: (a) NaH, THF, reflux; (b) DIPEA, THF, RT; (c) 1,1′-carbonothionyldipyri-din-2(1H)-one, DCM, RT; (d) silver trifluoroacetate, TEA, AcCN, reflux; (e) HATU, DIPEA, THF, RT; (f) (i) DCM, RT; (ii) HgO, S, EtOH, reflux.
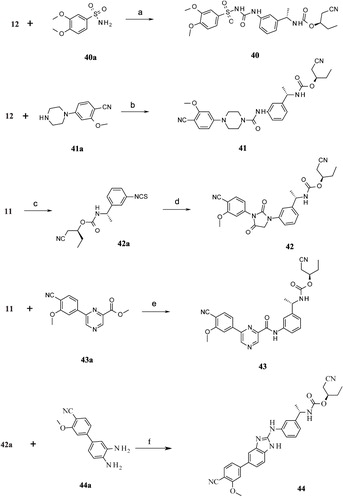
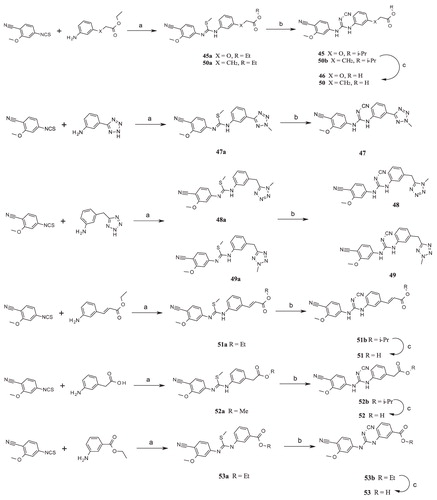
Scheme 5. Syntheses of compounds 45–53. Reagents and conditions: (a) (i) DCM, RT; (ii) MeI, K2CO3, RT; (b) NaNHCN, 2-propanol, reflux; (c) LiOH, THF:H2O (2:1), RT.
Scheme 6. Syntheses of compounds 54–60. Reagents and conditions: (a) 3-Bromoaniline, DCM, RT; (b) MeI, K2CO3, RT; (c) NaNHCN, 2-propanol, reflux; (d) substituted arylboronic acid, Cs2CO3, SPhos, Pd(OAc)2/EtOAc:toluene (1:1), 100 °C.
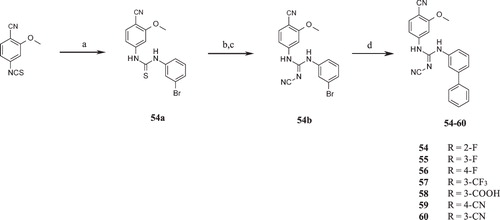
Table 2. In vitro inhibitory and cellular potencies of Part B modifications.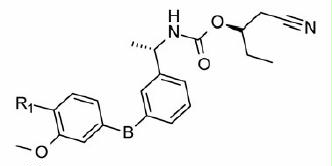
Table 3. In vitro inhibitory and cellular potencies of Part C modifications.