Figures & data
Scheme 1. Synthesis of N,N′-Bis[1-aryl-3-(piperidine-1-yl)propylidene]hydrazine dihydrochlorides, P1–P8. Reagents and conditions: (a) paraformaldehyde, piperidine HCl, HCl (37%) and EtOH, 4–9 h, for P1m, P3m–P8m; acetic acid (99%), 22 h, for P2m; (b) Ethanolic acetic acid (3% w/v), hydrazine hydrate for P1–P8. R1 = R2 = H (P1); R1 = CH3, R2 = H (P2); R1 = CH3O, R2 = H (P3); R1 = OH, R2 = H (P4); R1 = Cl, R2 = H (P5); R1 = H, R2 = CH3O (P6); R1 = F, R2 = H (P7); R1 = Br, R2 = H (P8).
![Scheme 1. Synthesis of N,N′-Bis[1-aryl-3-(piperidine-1-yl)propylidene]hydrazine dihydrochlorides, P1–P8. Reagents and conditions: (a) paraformaldehyde, piperidine HCl, HCl (37%) and EtOH, 4–9 h, for P1m, P3m–P8m; acetic acid (99%), 22 h, for P2m; (b) Ethanolic acetic acid (3% w/v), hydrazine hydrate for P1–P8. R1 = R2 = H (P1); R1 = CH3, R2 = H (P2); R1 = CH3O, R2 = H (P3); R1 = OH, R2 = H (P4); R1 = Cl, R2 = H (P5); R1 = H, R2 = CH3O (P6); R1 = F, R2 = H (P7); R1 = Br, R2 = H (P8).](/cms/asset/1186baa9-a356-45f3-b305-0353a5acd20d/ienz_a_795562_f0001_b.jpg)
Table 1. Experimental data of P series hydrazone compounds, P1–P8 ().
Table 2. Cytotoxic activity of hydrazones P1–P8 (), against Huh7 and T47D cells (IC50, μM).
Figure 1. In vitro effects of increasing concentrations of P7 on rat liver mitochondrial respiratory chain. p < 0.05 (one-way ANOVA followed by Tukey).
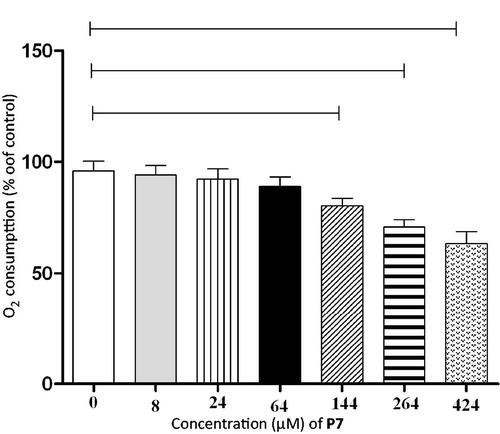
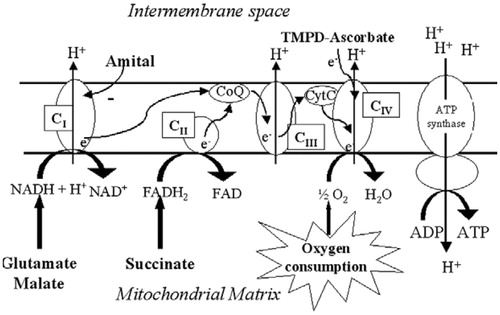
Figure 2. Schematic representation of the mitochondrial respiratory chain with specific substrates and inhibitors (modified from a previous studyCitation53). CI, complex I; CII, complex II; CIII, complex III; CIV, complex IV; TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride; H, proton.