Figures & data
Figure 1. Multialignment of the amino acid sequences of α-CAs from different sources was performed with the program MUSCLE. Legend: hCA II, Homo sapiens, isoform II (accession no. AAH11949.1); SspCA, Sulfurihydrogenibium sp. YO3AOP1 (accession no. ACD66216.1); SazCA, Sulfurihydrogenibium azorense (accession no. ACN99362.1); SupCA, super-CA (chimeric α-CA). The zinc ligands (His94, His96 and His119), the gatekeeper residues (Glu106 and Thr199) and the proton shuttle residue (His64) are conserved in all these enzymes and are indicated in bold. hCAI numbering system was used. Histidines in position 4, 17, 35, 41 and 48 were inserted to create the super-CA. The asterisk (*) indicates identity at all aligned positions. The symbol (:) relates to conserved substitutions, while (.) means that semi conserved substitutions are observed.
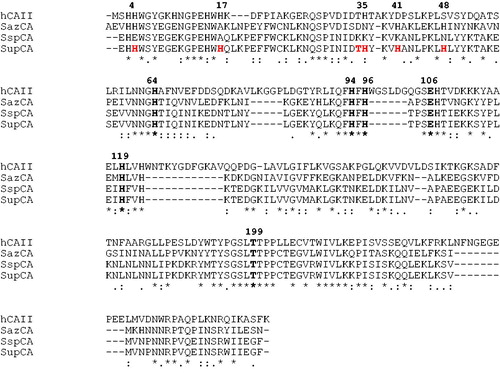
Figure 2. Phylogenetic tree of SupCA, SazCA, SspCA and hCA II. Tree was constructed using the program PhyML3.0. Sequence names and accession numbers are reported in .