Figures & data
Figure 1. Synthetic route for the preparation of six novel steroidal derivatives, 5a–5f and 6. (a) NaOH 10%, MeOH; (b) RCOOH, 4-dimethylaminopyridine (DMAP), N,N′-dicyclohexyl carbodiimide (DCC); (c) phosphorus oxychloride (POCl3), dimethyl formamide (DMF), CHCl3; reflux, 5 h; (d) Benzimidazole, Na2CO3, DMF; (e) Formic acid, reflux, 5 h; (f) 1. Benzimidazole, Cs2CO3, DMF, 60 °C, 2 h; 2. CHCl3/MeOH, HCl, 40 °C, 3 h.
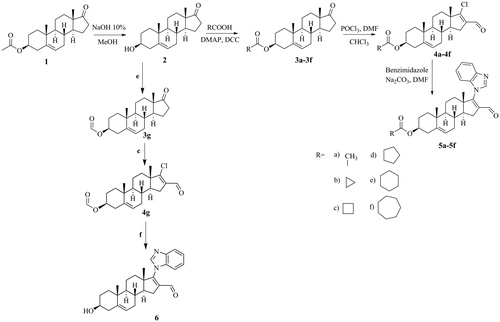
Figure 2. Biological activities of finasteride and the six novel steroidal derivatives 5a–5f and 6. IC50: concentration of compound required to inhibit 50% of the activity of 5α-reductase isoenzymes 1 and 2.
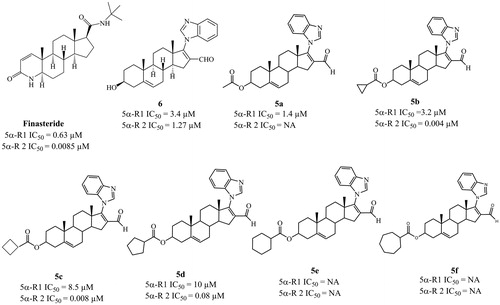
Figure 3. Diameters of the pigmented spot (±standard deviation) of flank organs of castrated and intact (C) hamsters. For 6 d, the castrated hamsters received subcutaneous injections of the various test materials, with the animals treated with vehicle only (V). The experiment was carried out in duplicate. The asterisks show the instances of statistically significant difference (p < 0.05) between the group of hamsters treated with testosterone (T) and those treated with T and with either finasteride (F) or with the synthesized steroids (5a–5f, and 6 see for structures).
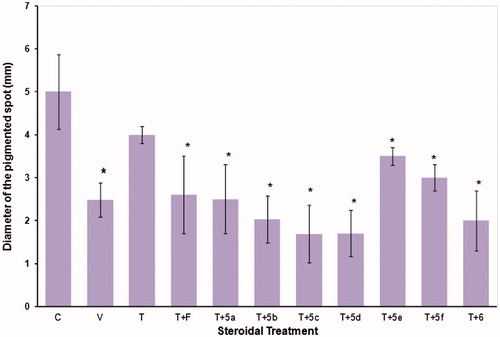
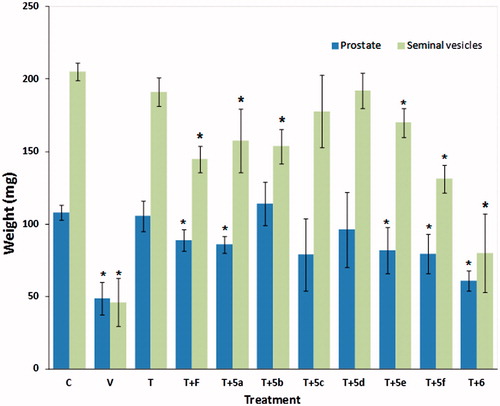
Figure 4. Weight of prostate and seminal vesicles glands (± standard deviation) from castrated and intact animals (C) hamsters. For 6 d, the castrated hamsters received different subcutaneous injections of the various test materials, with the castrated control animals (V) treated with vehicle only. The experiment was carried out in duplicate. The asterisks show the instances of statistically significant difference (p < 0.05) between the group of hamsters treated with testosterone (T) and those treated with T and with either finasteride (F) or with the synthesized steroids (5a–5f, and 6 see for structures).