Figures & data
Figure 1. The chemical structure of phenolic compounds, including arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2,4,6-trihydroxybenzaldehyde as inhibitors for hCA I and II isozymes.
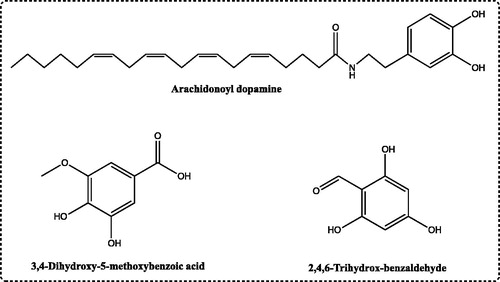
Figure 2. Elution graph of the hCA I and II isoenzymes purified by using Sepharose-4B-L-Tyrosine affinity chromatography.
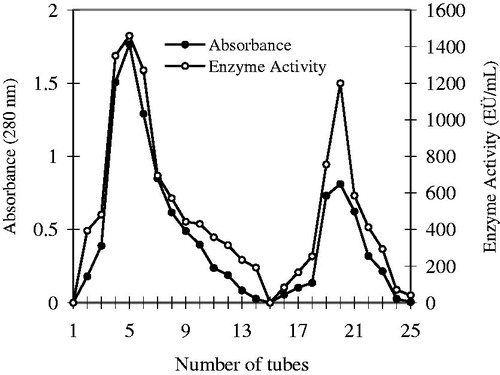
Figure 3. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified CA I and II isozymes from human erythrocytes. Lane A and D: standard proteins (kD): β-galactosidase from E. coli (116), phosphorylase B from rabbit muscle (97), bovine serum albumin (66), chicken ovalbumin (45) and carbonic anhydrase (29). Lanes B and F: human carbonic anhydrase I. Lanes C and E: human carbonic anhydrase II.
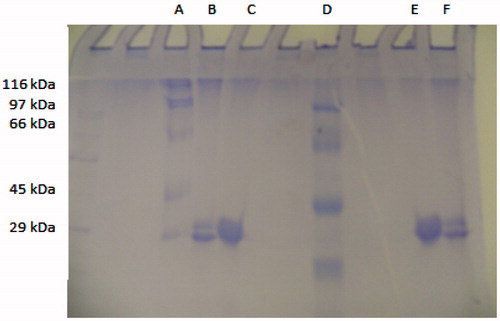
Table 1. Summary scheme of hCA I and II isoenzymes purified using Sepharose-4B-L-tyrosine affinity chromatography.
Table 2. The IC50, Ki and inhibition type of phenolic compounds for the hCA I and II isozymes as ex vivo using the esterase activity method.
Table 3. The IC50 values of substances for the hCA I and II isozymes ex vivo using the CO2-hydratase activity method.
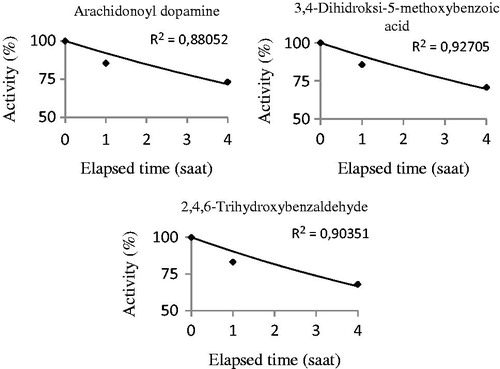
Figure 4. The half-life (t50) values of the substances were calculated by activity%-elapsed time graphs in in vivo studies. The half-life (t50) values were found for arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2,4,6-trihydroxybenzaldehyde. These experiments were performed using CO2-hydratase activity assays.