Figures & data
Figure 1. Electrophoretic analysis of different chitosan/pDNA complexes and free plasmid. Lane 1: DNA ladder. Lanes 2, 10 and 18: pDNA. Lanes 3–9, 11–17, 19–25: Chitosan/pDNA complexes of 18, 50 and 136 KD, respectively at varying concentrations.
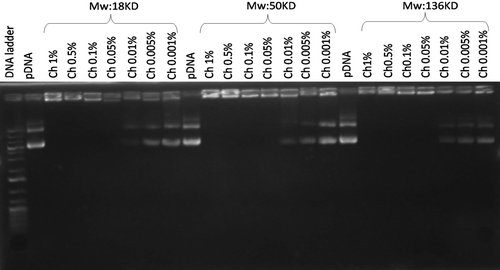
Figure 2. SEM image of chitosan/pDNA complex. Representative image is the 18 KD chitosan at 0.1% concentration.
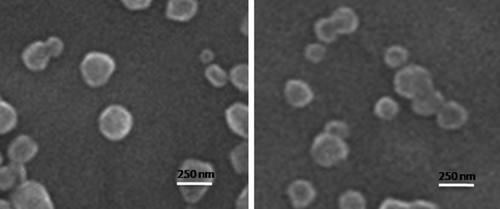
Table I. Molecular weight, concentration, particle size and zeta potential of chitosan nanoparticles.
Figure 3. Human MSC culture and differentiation. (A) Bone marrow cell at primary culture. (B) Confluent culture of passaged-3 cells. (C) Alizarin red staining of the osteogenic culture. (D) The same osteogenic culture without staining. (E) Oil red staining of the adipogenic culture. (F) Adipogenic culture without staining. (G) Toluidine blue staining of chondrogenic culture.
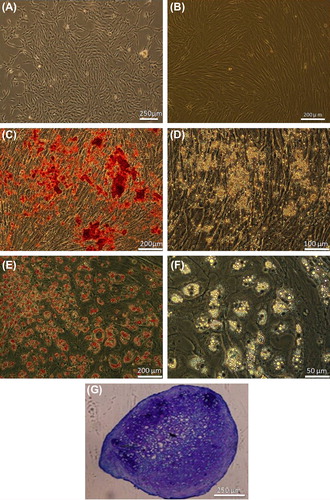
Figure 4. pH optimization for MSC transfection. Four different concentrations of 18 KD chitosan were used to determine the optimal pH for maximum transfection of human marrow MSC. *p < 0.01.
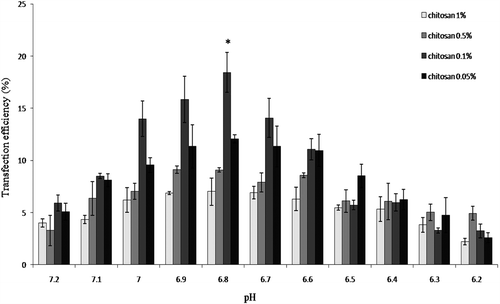
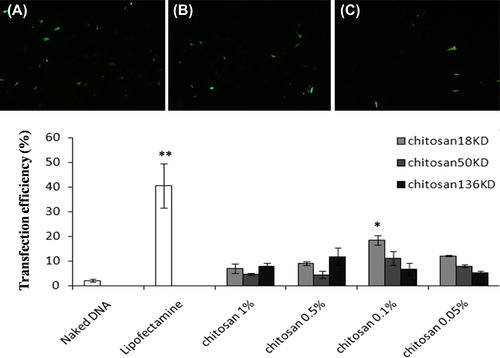
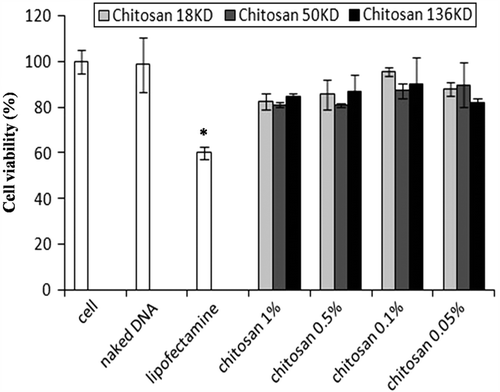
Figure 5. Human MSC transfection with chitosan/pDNA nanoparticles. Transfected cells appear green as visualized by fluorescent microscope. (A) Commercial lipofectamine group. (B) Nanoparticle group (18 KD chitosan/pDNA at 0.1% concentration). (C) Naked DNA (original magnification: 10x). (D) Commercial lipofectamine tended to yield better transfection than nanoparticles. **p < 0.01. Nanoparticles produced significantly more transfection than naked DNA. Comparatively, nanoparticles formed by 18 KD chitosan at the 0.1% concentration tended to have more transfection efficacy than the other studied nanoparticles. *significant difference (p < 0.01).