Figures & data
Table 1a. Chemical properties of NMO
Table 1b. Physical properties of NMO
Table 2. Physicochemical properties and NOx conversion over NMO and V/NMO
Figure 2. Effect of V loading and OA addition on NOx conversion over NMO (space velocity [SV] = 60,000 hr−1, NO = 190 ppm, NO2 = 20 ppm, water vapor = 8%, O2 = 15%, NOx/NH3 = 1).
![Figure 2. Effect of V loading and OA addition on NOx conversion over NMO (space velocity [SV] = 60,000 hr−1, NO = 190 ppm, NO2 = 20 ppm, water vapor = 8%, O2 = 15%, NOx/NH3 = 1).](/cms/asset/78ca07ec-b93f-4738-85cd-6ca379395e74/uawm_a_10412121_o_f0002g.gif)
Figure 3. Effect of temperature on NH3 oxidation over NMO and V/NMO (SV = 60,000 hr−1, NH3 = 225 ppm, water vapor = 8%, O2 = 15%, NO = 0, NO2 = 0).
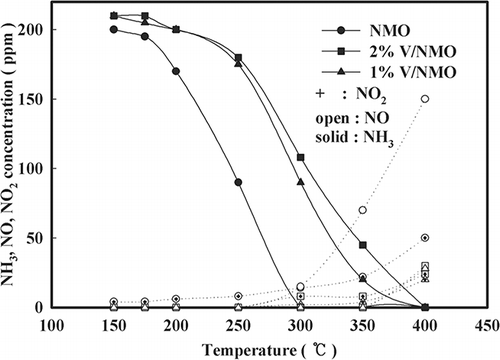
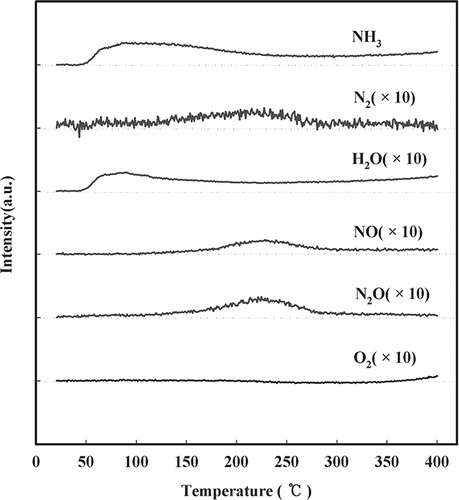
Figure 4. Effect of temperature on NH3 oxidation over NMO as detected by mass spectroscopy (SV = 60,000 hr−1, NH3 = 225 ppm, water vapor = 8%, O2 = 15%, NO = 0, NO2 = 0).
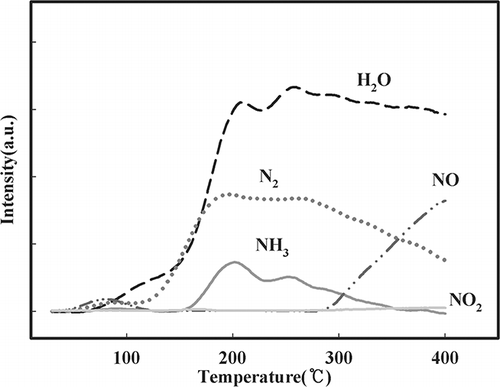
Figure 5. Effect of temperature on NO oxidation over NMO and V/NMO (SV = 60,000 hr−1, NO = 190 ppm, NO2 = 20 ppm, water vapor = 8%, O2 = 15%).
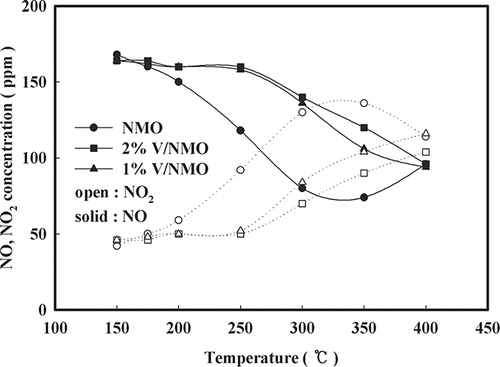
Figure 7. Comparison of NOx conversion with all desorbed nitrogen compounds from (a) B and (b) total acid sites over various samples.
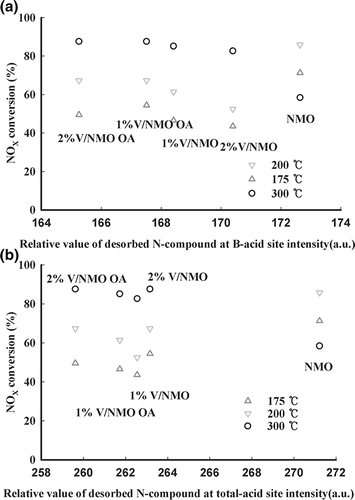
Figure 8. Comparison of NOx conversion with all desorbed nitrogen compounds from L acid sites over various samples.
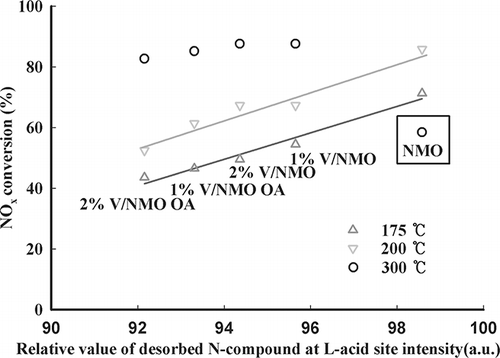
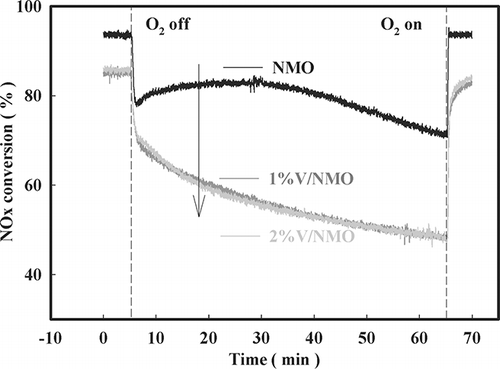
Figure 9. Decrease in NOx conversion with time after O2 on-off over NMO (SV = 60,000 hr−1, NO = 190 ppm, NO2 = 20 ppm, water vapor = 8%, O2 = 15%, NOx/NH3 = 1).
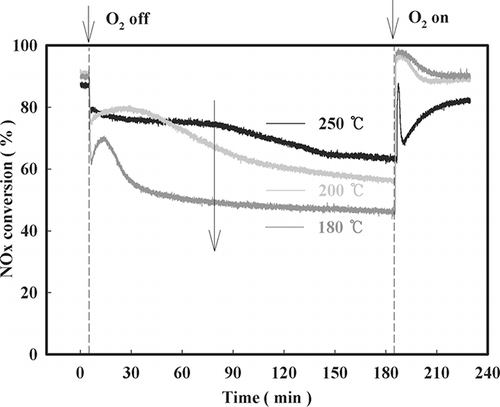
Figure 10. Decrease in NOx conversion with time after O2 on-off over NMO, 1%V/NMO, and 2%V/NMO (SV = 60,000 hr−1, NO = 190 ppm, NO2 = 20 ppm, water vapor = 8%, O2 = 15%, NOx/NH3 =1 at 200 °C).