Figures & data
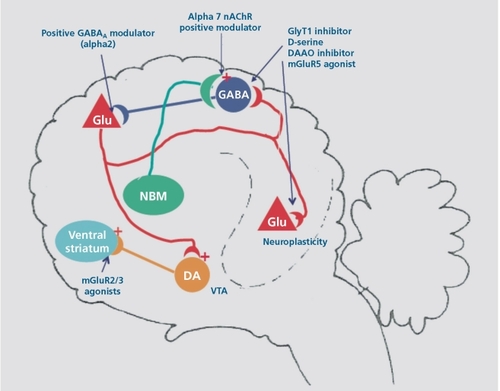
Figure 1. Schematic representation of the synaptic circuitry relevant to the pathophysiology of schizophrenia. NMDA receptor hypofunction can be produced by exogenous antagonists such as ketamine, endogenous antagonists such as N-acetyl aspartyl glutamate (NAAG) or kyneurenic acid, reduced availability of D-serine due to increased activity of D-amino acid oxidase (DAAO) or mutant NR2B. This results in dendritic dysplasia on pyramidal neurons and reduced activity of the parvalbumin positive GABAergic interneurons. Reduced recurrent inhibition disrupts cortical processing, causing cognitive impairment and negative symptoms and increased excitatory drive to the ventral tegmental area (VTA), leading to psychosis. An allelic variant of the gene encoding the α7 nicotinic receptor causes reduced expression and disrupts sensory gating. NMDA, N-methylD-aspartate; GABA, γ-aminobutyric acid; DA, dopamine; NBM, nucleus basalis of Meynert; nAchR, nicotinic acetylcholine receptor; Glu, glutamate
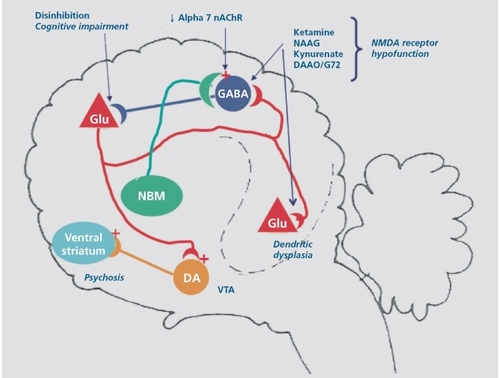
Figure 2. Potential pharmacologic interventions to treat schizophrenia: (i) Enhance NMDA receptor function by increasing synaptic glycine concentrations with an inhibitor of GlyT1 , administering exogenous D-serine, inhibiting D-amino acid oxidase or by treating with an mGluR5 agonist that augments NMDA receptor function; (ii) Increase the excitability of the parvalbumin-positive GABAergic interneurons with a c 7nicotine receptorpositive modulator; (iii) Reduce pyramidal neuron excitability with GABAA receptor-positive modulator. (iv) Decrease disinhibited pyramidal neuron glutamate release with an mGluR2/3 agonist. NMDA, N-methyl-D-aspartate; GABA, γ-aminobutyric acid; DA, dopamine; NBM, nucleus basalis of Meynert; mAchR, metabotrophic acetylcholine receptor; Glu, glutamate; DAAO, D-amino acid oxidase