Figures & data
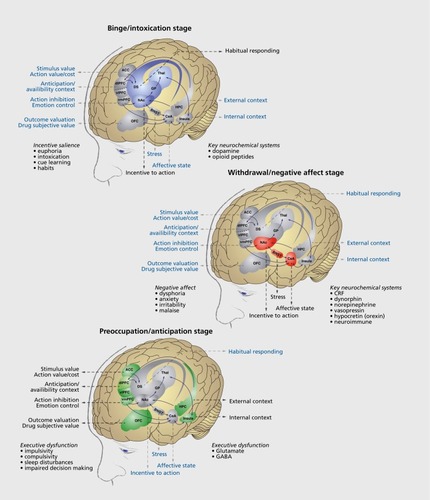
Figure 3 (Opposite) Neural circuitry associated with the three stages of the addiction cycle. (A)
Binge/intoxication stage. Reinforcing effects of drugs may engage associative mechanisms and reward neurotransmitters in the nucleus accumbens shell and core and then engage stimulusresponse habits that depend on the dorsal striatum. Two major neurotransmitters that mediate the rewarding effects of drugs of abuse are dopamine and opioid peptides. (B)
Withdrawal/negative affect stage. The negative emotional state of withdrawal may engage the activation of the extended amygdala. The extended amygdala is composed of several basal forebrain structures, including the bed nucleus of the stria terminalis, central nucleus of the amygdala, and possibly a transition area in the medial portion (or shell) of the nucleus accumbens. Major neurotransmitters in the extended amygdala that are hypothesized to play a role in negative reinforcement are corticotropinreleasing factor, norepinephrine, and dynorphin. The extended amygdala has major projections to the hypothalamus and brain stem. (C)
Preoccupation/anticipation (craving) stage. This stage involves the processing of conditioned reinforcement in the basolateral amygdala and processing of contextual information in the hippocampus. Executive control depends on the prefrontal cortex and includes the representation of contingencies, the representation of outcomes, their value, and subjective states (ie, craving and, presumably, feelings) associated with drugs. The subjective effects, termed drug craving in humans, involves activation of the orbitofrontal and anterior cingulate cortex and temporal lobe, including the amygdala, in functional imaging studies. A major neurotransmitter that is involved in the craving stage is glutamate that is localized in pathways from frontal regions and the basolateral amygdala that project to the ventral striatum. ACC, anterior cingulate cortex; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CRF, corticotropin-releasing factor; dlPFC, dorsolateral prefrontal cortex; DS, dorsal striatum; GABA, γ-aminobutyric acid; GP, globus pallidus; HPC, hippocampus; NAc, nucleus accumbens; OFC, orbitofrontal cortex; Thai, thalamus; vIPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex. Modified with permission from reference 14: Koob GF, Everitt BJ, Robbins TW. Reward, motivation, and addiction. In: Squire LG, Berg D, Bloom FE, Du Lac S, Ghosh A, Spitzer N, eds. Fundamental Neuroscience. 3rd edition. Amsterdam, the Netherlands: Academic Press; 2008:987-1016. Copyright © Academic Press, 2008