Figures & data
Table 1 Size parameters of different beans
Fig. 1 Epi-illumination micrographs of tegument transverse sections from four varieties of beans. On the right of each image, a two-dimensional graph of the pixel value as a function of the distance along the yellow line is displayed.
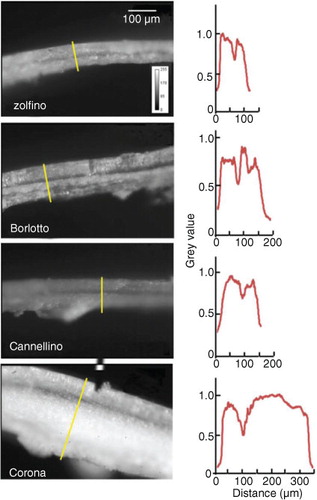
Fig. 2 Water entry and extrusion of molecular species. Beans were suspended (0.4 g/mL) in deionized water and left at room temperature (22–25°C). Panel a: at the indicated times, the incubation medium was carefully removed and the water entry, reported as a percentage of maximal imbibition, was evaluated by the increase in bean weight. Symbols (![]()
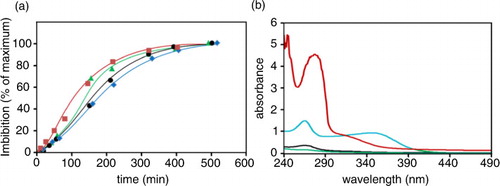
Fig. 3 Time course of chromophores extrusion during beans imbibition. The release of bean components and incubation times are reported for different beans as the absorbance measured at distinctive wavelengths, namely 265 (![]()
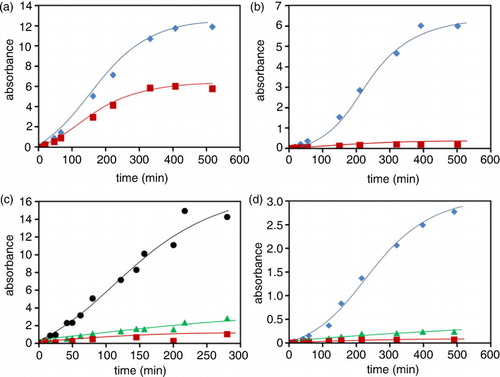
Fig. 4 Yellow chromophores from Zolfino. Panel a: Absorption spectra of methanol extract of Zolfino tegument (the tegument from 1.5 g of seeds soaked in 3 mL methanol for 1 h at room temperature) (red line) and of 20 µM in methanol of authentic kaempferol-3O-glucoside (black line) are reported. Panel b: absorption spectra of water extract from tegument (green line), cotyledons (red line), and whole (blue line) Zolfino bean are reported. Teguments and cotyledons derived from 1.5 g of Zolfino seeds were soaked for 1 h at room temperature in 3 and 6 mL of water, respectively, and the medium analyzed. Whole seeds (1.5 g) were soaked as above in 6 mL of water and the medium analyzed.
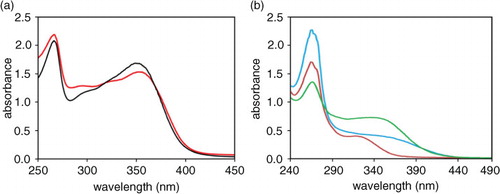
Fig. 5 Electrophoretic pattern of water-soluble proteins from different bean varieties. Water-soluble proteins extracts from bean flours (100 mg/mL) were analyzed by SDS-PAGE on 12% acrylamide gels. 3 µg of proteins were applied to each well. Panel a: Coomassie blue–stained gel of water flour extracts of Zolfino, Cannellino, Borlotto, and Corona beans (lanes 2 to 5, respectively). Lane 1 refers to MW standard proteins. Numbers alongside the gel represent their apparent molecular weights divided by 1,000. Panel b: molecular-weight calibration curve using standard proteins (lane 1 of the stained gel). The equation reported was obtained by linear regression analysis of standards migration Panel c: aligned electropherogram profiles for Zolfino (a), Cannellino (b), Borlotto (c), and Corona (d). Arrows marked with letters a–h refer to the migration of MW standards reported in Panel b. Numbers on the profiles refer to specific protein bands characterizing the protein patterns. The letters O and F refer to the origin and to the front of the migration, respectively.
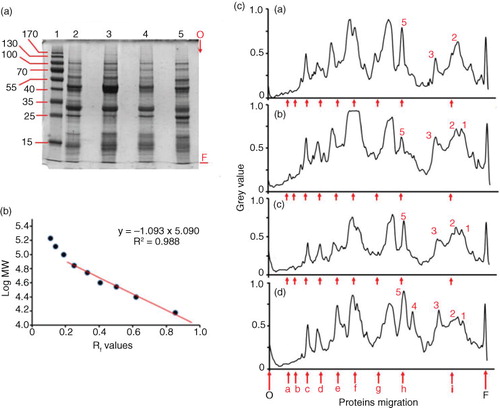
Table 2 Some nutritional parameters of different beans
Fig. 6 Polyol pathway enzymes inhibition by bean water extracts. The effect of different extracts from Zolfino (![]()
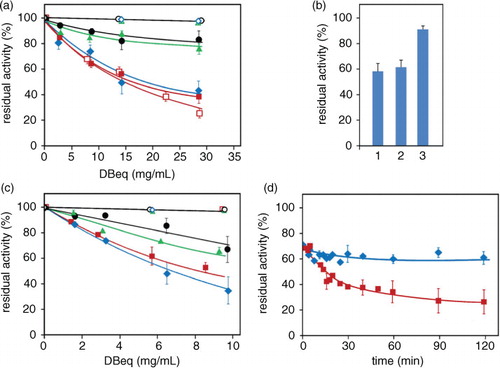
Fig. 7 Aldose reductase inhibition by water extract of bean flours. The effect of different extracts from the flour of Zolfino (![]()
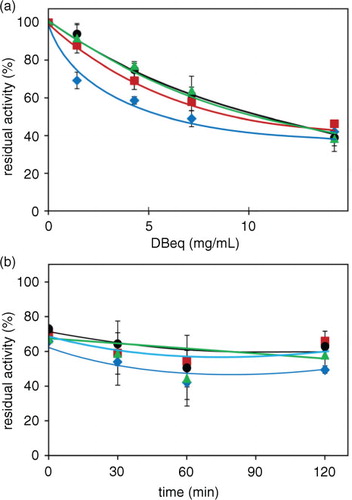
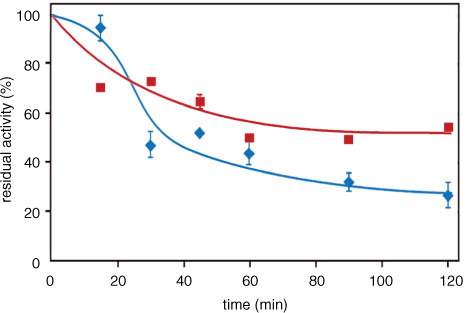
Fig. 8 Aldose reductase inhibition by the ‘cooking media’ of Zolfino and Borlotto. The suspending media of Zolfino directly incubated at 100°C (400 mg/mL) in water, and of Borlotto incubated as above after the removal of the water in which the bean (400 mg/mL) had been soaked overnight at room temperature. Residual AR activity was measured at different cooking times using 8.6-DBeq mg/mL and is reported as a percentage of the activity in the absence of the effector, which accounts for 8 mU. Error bars (when not visible, these are within the symbol size) represent the standard deviation from three independent measurements. (![]()