Figures & data
Fig. 1. N2a cells constitutively expressing GFP-CD63 (N2aGFP-CD63) secrete exosomes containing the fusion protein. (A) Co-staining of N2aGFP-CD63 with anti-LBPA (red) shows that GFP-CD63 (green) is concentrated inside LBPA-containing multivesicular bodies (arrows). (B) Density separation of extracellular vesicles released by N2aGFP-CD63 cells; Western blot analysis using anti-GFP, anti-flotillin-1 and anti-Alix, shows GFP immunoreactivity in fractions containing exosomes. TCL: total cell lysates, Inp: input. (C, D) Immunogold labelling of vesicles secreted by N2aGFP-CD63 cells pelleted at 100,000×g. Anti-CD63 labels the surface of intact exosomes (C), while anti-GFP stains the lumen of permeabilized exosomes (D). Scale bars: (A) 5 µm, (C, D) 100 nm.
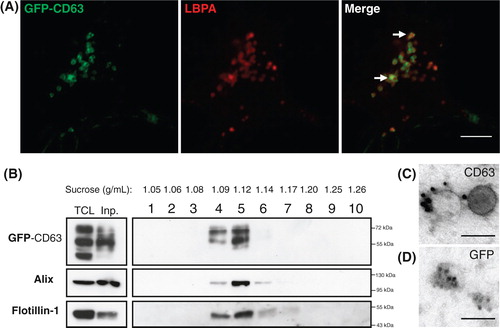
Fig. 2. Soluble GFP–TTC is endocytosed before being secreted via neuronal exosomes. Cortical neurons were incubated at 37°C with GFP–TTC for 2 h (36 nM). (A) Confocal microscopy of neurons stained with anti-EEA1 (red), shows that GFP–TTC proteins (green) bind to and are endocytosed by neurons. (B) Density separation of extracellular vesicles secreted during a 15 min treatment with bicuculline and 4-AP; Western blot analysis using anti-GFP, anti-flotillin-1 and anti-Alix, shows GFP immunoreactivity in fractions containing exosomes. TCL: total cell lysates, Inp: input. (C) Immunogold labelling with anti-GFP of vesicles pelleted at 100,000×g demonstrates the presence of GFP–TTC on the exosomal surface. Scale bars: (A) 10 µm (C) 100 nm.
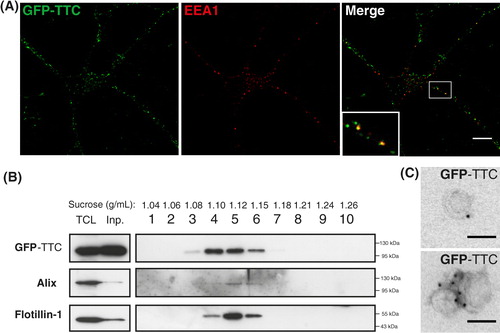
Fig. 3. Neuroblastoma exosomes carrying GFP-CD63 bind to and are endocytosed by neurons and glial cells. Exosomes released by N2aGFP-CD63 cells were resuspended in conditioned medium and incubated for 1 h on mixed primary culture of hippocampal neurons (14 DIV). After washing, cells were co-stained with antibodies against MAP2 (A), or GFAP (B), or O4 (C), to label neurons, astrocytes and oligodendrocytes, respectively, and with antibodies against Lamp-1 (A, B, C) to stain late endosomes and lysosomes. Arrows point to exosomes bound on the cell surface. Arrowheads point to colocalization of exosomes with Lamp-1. Stacks of 3 confocal sections inside cells are shown for all conditions. Scale bars: 10 µm.
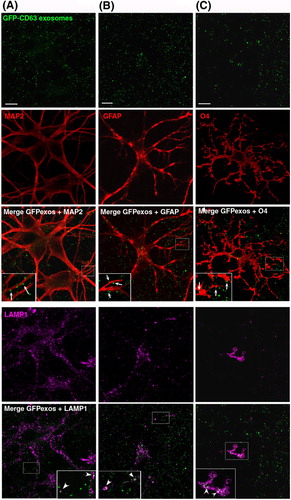
Fig. 4. Neuronal exosomes bearing GFP–TTC bind specifically to neurons. (A) Exosomes released by cortical neurons pre-incubated with GFP–TTC were harvested, pelleted at 100,000×g and separated on a sucrose gradient. Three GFP–TTC containing fractions (sucrose density of 1.1–1.15 g/ml) were pooled, pelleted, resuspended in incubation medium and incubated for 1 h on hippocampal cell cultures (16 DIV) (A, B, C). A) After washing, cells were immunostained with anti-MAP2 antibody (red) to label neurons. Hoechst nuclear staining shows the presence of numerous MAP2 negative cells which are not stained with GFP–TTC exosomes (maxima intensity). (B) After washing, cells were immunostained with anti-MAP2 antibody (red) to label neurons and anti-GFAP to stain astrocytes (magenta). (C) Cells were washed and processed for immunogold labelling using anti-GFP and processed for EM observation. Single or aggregated exosomes carrying gold-labelled GFP–TTC can be seen on the surface of neurons. Scale bars: (A, B) 10 µm, (C) 500 nm.
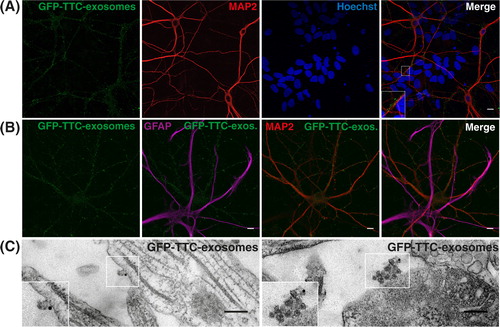
Fig. 5. Neuronal exosomes bearing GFP–TTC preferentially bind to pre-synaptic terminals and can be internalized. Exosomes secreted by cortical neurons preincubated with GFP–TTC (green) were incubated for 1 h on hippocampal neurons (22 DIV), which were then immunostained with (A) anti-synaptophysin (magenta) or (B) anti-PSD95 (magenta). Confocal microscopy sections show patches of GFP–TTC exosomes (green) perfectly colocalized with some synaptophysin-positive presynaptic sites and juxtaposed with some PSD95-positive post-synaptic sites. Scale bars: (A, B, C) 10 µm. (C) GFP–TTC exosomes were incubated for 1 h on hippocampal neurons (21 DIV) preincubated with Alexa594-WGA for 10 min at 37°C to label endosomes. (A), (B) (C) are confocal sections except for the photo on the right of the (C) panel, representing the projection of maximal intensities. Thin and bold arrows show labelling on the surface and within the cytoplasm respectively.
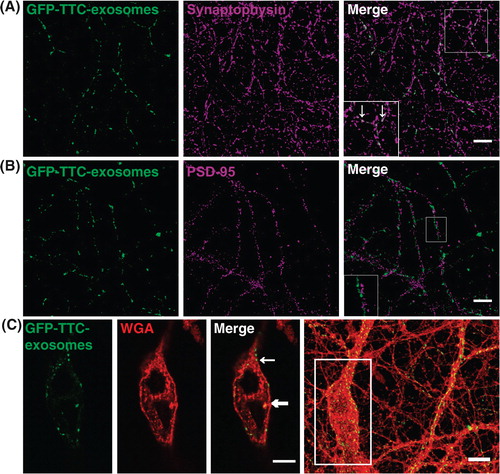
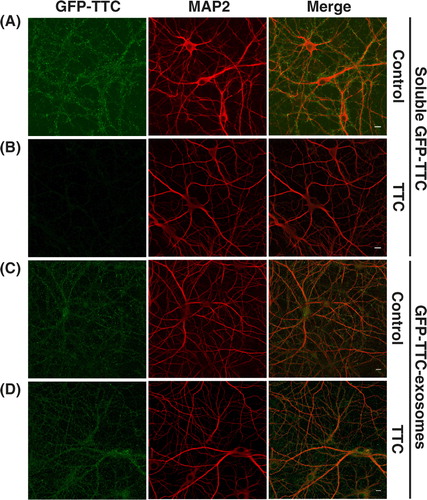
Fig. 6. GFP–TTC exosomes do not bind to the neuronal surface because of their TTC cargo. (A, B) Incubation with TTC abolishes GFP–TTC staining of neurons: GFP–TTC was diluted to 0.36 nM in culture medium and incubated for 1 h on 16 DIV hippocampal neurons in absence (A) or in presence (B) of 100 nM TTC. In B) cells were pre-incubated for 20 min with 100 nM TTC. (C, D) TTC does not impair binding of GFP–TTC-exosomes to neurons: GFP–TTC-exosomes were incubated for 1 h on 16 DIV hippocampal neurons in absence (C) or in presence (D) of 100 nM TTC. In D) cells were pre-incubated for 20 min with 100 nM TTC. After incubation, cells were washed, fixed and immunolabelled with anti-MAP2 (red) (A, B, C and D). Scale bars: 10 µm.