Figures & data
Table I. Current and past NIH registered clinical trials investigating EV-based therapeutics
Fig. 1. Pharmaceutical categories and a suggested classification of EV-based therapeutics. Chart depicts the Categories of Medicinal Products with respect to their origin (chemical, biological, herbal). Medicinal Products (according to DIRECTIVE 2001/83/EC) include any substance or combination of substances for treating or preventing disease in humans. Any substance or combination of substances which may be administered to humans with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions in humans is likewise considered a medicinal product. The suggested classification of EV-based therapeutics within the class of biological medicinal products is provided (grey fields). Bold indicates categories from which existing legislation is recommended to be considered for preclinical and clinical development of EV-therapeutics.
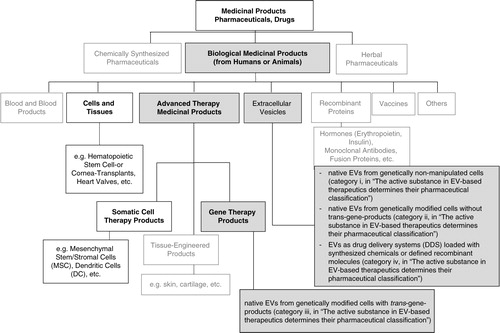
Table II. To be defined and established by investigators before (or concomitant to) clinical application of EV-based therapeutics
