Figures & data
Figure 1. The drug testing dilemma.
The graph illustrates schematically the route from substance development through preclinical and clinical evaluation of toxicity and efficacy until release. The process is estimated to take an average of 13.5 years. Depending on the source, only 4–10% of the initial drug candidates will become market-ready and will cross-finance the withdrawn substances. Retraction at a late stage becomes especially costly, which is why the developing companies try to improve the predictability in the preclinical phases and resolve inapt drugs (numbers are extracted from Paul et al. [Citation1]).
![Figure 1. The drug testing dilemma.The graph illustrates schematically the route from substance development through preclinical and clinical evaluation of toxicity and efficacy until release. The process is estimated to take an average of 13.5 years. Depending on the source, only 4–10% of the initial drug candidates will become market-ready and will cross-finance the withdrawn substances. Retraction at a late stage becomes especially costly, which is why the developing companies try to improve the predictability in the preclinical phases and resolve inapt drugs (numbers are extracted from Paul et al. [Citation1]).](/cms/asset/59ef14f3-e73b-4e4f-a611-63f9e8b0c28e/ifso_a_12363969_f0001.jpg)
Figure 2. Selection of current microphysiological systems devices with ongoing marketing or commercialization ambitions.
The first row shows sophisticated single-organ-specific chip designs. The second row depicts pumpless plate systems with a broad range of applications through a generic layout. The bottom row illustrates multi-organ-chips with individualized cultivation compartments. (A) The lung-on-a-chip hosts a barrier model containing lung epithelial and endothelial cells (reprinted with permission from Huh et al. [Citation24]). (B) The LiverChip® incorporates primary human liver cells showing extended vitality and in vivo-like morphogenesis over prolonged culture periods (reprinted with permission from Domansky et al. [Citation89]). (C) The OrganoPlates® allow the controlled deposition and subsequent perfusion of hydrogels (reprinted with permission from Wevers et al. [Citation46]). (D) The microfluidic platform accommodates up to eight cell aggregates in compartments that allow communication through the medium (courtesy of Oliver Frey). (E) The body-on-a-chip device designed based on a PBPK model adapts compartment size and microfluidics to the human template considering technological constraints (reprinted with permission from Miller and Shuler [Citation56]). (F) The Four-Organ-Chip was manufactured to mimic adsorption, distribution, metabolism and excretion – information mandatory for the toxic evaluation of any given substance.
PBPK: Physiologically based pharmacokinetic.
![Figure 2. Selection of current microphysiological systems devices with ongoing marketing or commercialization ambitions.The first row shows sophisticated single-organ-specific chip designs. The second row depicts pumpless plate systems with a broad range of applications through a generic layout. The bottom row illustrates multi-organ-chips with individualized cultivation compartments. (A) The lung-on-a-chip hosts a barrier model containing lung epithelial and endothelial cells (reprinted with permission from Huh et al. [Citation24]). (B) The LiverChip® incorporates primary human liver cells showing extended vitality and in vivo-like morphogenesis over prolonged culture periods (reprinted with permission from Domansky et al. [Citation89]). (C) The OrganoPlates® allow the controlled deposition and subsequent perfusion of hydrogels (reprinted with permission from Wevers et al. [Citation46]). (D) The microfluidic platform accommodates up to eight cell aggregates in compartments that allow communication through the medium (courtesy of Oliver Frey). (E) The body-on-a-chip device designed based on a PBPK model adapts compartment size and microfluidics to the human template considering technological constraints (reprinted with permission from Miller and Shuler [Citation56]). (F) The Four-Organ-Chip was manufactured to mimic adsorption, distribution, metabolism and excretion – information mandatory for the toxic evaluation of any given substance.PBPK: Physiologically based pharmacokinetic.](/cms/asset/85f8aa6e-ef94-4c6f-9b90-8ae4ab9883dc/ifso_a_12363969_f0002.jpg)
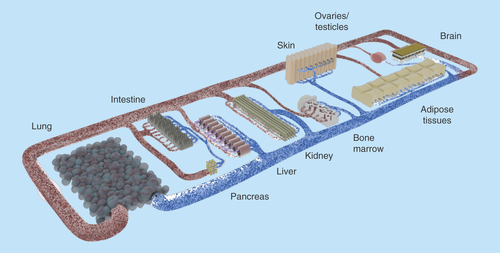
Figure 3. Concept of a human-on-a-chip.
The pursuit of the most important bodily functions will lead to a miniature organism on a chip. Common tests conducted in rodents should be realizable with this device. Therefore, the products of the organoids (e.g., urine) need to be discharged in separate compartments. Oral, dermal and intravenous uptake routes, and through inhalation, need to be possible. The transparent device enables optical analysis. Incorporated electrodes will assess barrier resistance, electrophysiological data and key parameters in the supernatants.