Figures & data
Figure 1. Cisplatin (CP) activates AKT and Chk1 in OS cells. (A) OS cells lines were treated with CP (5 μM for SJSA1 and MG63, 10 μM for MHM and U2OS, 2 μM for SAOS) for 48 hours. Whole cell lysates were immunoblotted for pAKT (S473), total AKT, pChk1 (S345), Chk1, p53, and β-actin. (B) The cells were treated with indicated doses of CP for 72 hours and analyzed with FACS for sub-G1 apoptosis. Average percent apoptotic cells from triplicate were presented as a graph with standard deviation indicated. (Representative of at least 3 independent experiments).
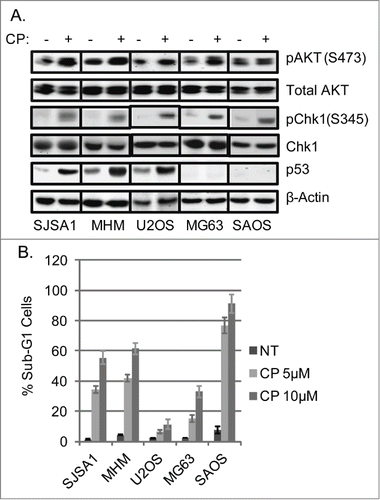
Figure 2. Chk1 inhibitor sensitizes OS cells to cisplatin. (A) OS cells lines were treated with CP (5 μM for SJSA1 and MG63, 10 μM for MHM and U2OS, 2 μM for SAOS) for 48 hours. Whole cell lysates were immunoblotted for pChk1 (S296 and S345), Chk1, p53, and β-actin. (B) The cells were treated with CP (5 μM for SJSA1 and MG63, 10 μM for MHM, 15 μM for U2OS, 2 μM for SAOS) and LY2603618 (LY, 0.2 μM) singly or combined for 72 hours and analyzed with FACS for sub-G1 apoptosis. Average percent apoptotic cells from triplicate were presented as a graph with standard deviation indicated. There is significant difference between CP and CP plus LY (p < 0.001) in all the cell lines. (Representative of at least 3 independent experiments).
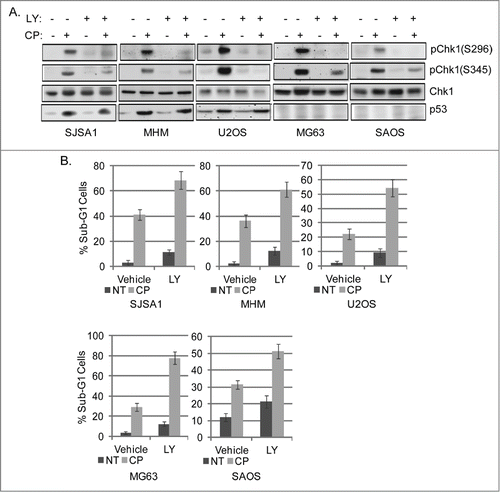
Figure 3. Inhibition of AKT increases CP-induced apoptosis in OS cells expressing WT p53 but not p53-null cells. (A) OS cells lines were treated with CP alone (5 μM for SJSA1 and MG63, 10 μM for MHM and U2OS, 2 μM for SAOS) or CP plus MK2206 (MK, 10 μM) for 48 hours. Whole cell lysates were immunoblotted for pAKT (S473), p53, and β-actin. (B) The cells were treated with CP and MK2206 (doses as above) singly or combined for 72 hours and analyzed with FACS for sub-G1 apoptosis. Average percent apoptotic cells from triplicate experiments is graphed with standard deviation indicated. There is significant difference between CP and CP plus MK (p < 0.01) in SJSA1, MHM, and U2OS cell lines and no significant difference (p > 0.5) in MG63 and SAOS cells. (C) U2OS cells expressing control shRNA (Csh) or p53 shRNA (p53sh) were treated with 15 μM CP for 48 hours and whole cell lysates immunoblotted for p53 and β-actin. (D) U2OS cells expressing control shRNA (Csh) or p53 shRNA (p53sh) were treated with 15 μM CP and 10 μM MK singly or combined for 72 hours and analyzed with FACS for sub-G1 apoptosis. Average percent apoptotic cells from triplicate were presented as a graph with standard deviation indicated. There is significant difference between CP and CP plus MK (p < 0.01) in Csh cells but not in p53 sh cells (Representative of at least 3 independent experiments).
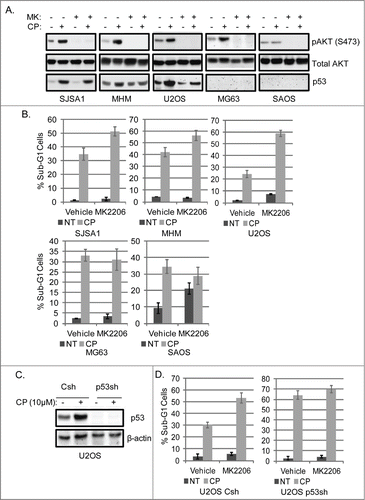
Figure 4. P53 knockdown reduces Noxa and Puma gene expression in cells treated with CP or CP plus MK2206. (A) MHM cells were transiently transfected with control siRNA (Csi) or p53 siRNA (p53si) were treated with CP (10 μM) or CP plus MK for 48 hours. Relative Noxa and Puma gene expression was analyzed by qTPCR and presented as graphs. (B) Whole cell lysates were immunoblotted for p53 and β-actin. (C) U2OS cells expressing control shRNA (Csh) or p53 shRNA (p53sh) were treated with CP (15 μM) or CP plus MK for 48 hours. Relative Noxa and Puma genes were analyzed by qTPCR and presented as graphs. (D) Whole cell lysates were immunoblotted for p53 and β-actin.
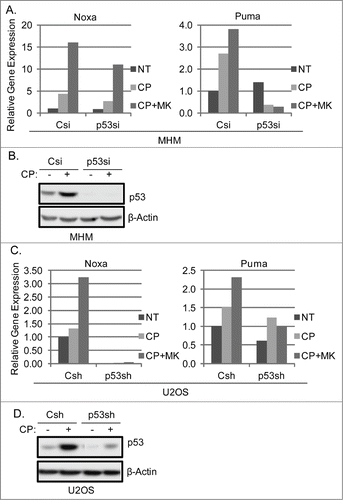
Figure 5. Knockdown of Noxa and Puma reduces MK2206 sensitization of OS cells to CP. MHM and U2OS cells were transfected with control siRNA (Csi), Noxa siRNA (Noxasi), or Puma siRNA (Pumasi) and treated with CP (10 μM for MHM, 15 μM U2OS) or CP plus MK. 72 hours later cells were analyzed for apoptosis (A, C and E). Cells were also harvested after 48 hour treatment and analyzed by immunoblotted for Noxa protein (B) or Noxa and Puma gene expression (D and F).
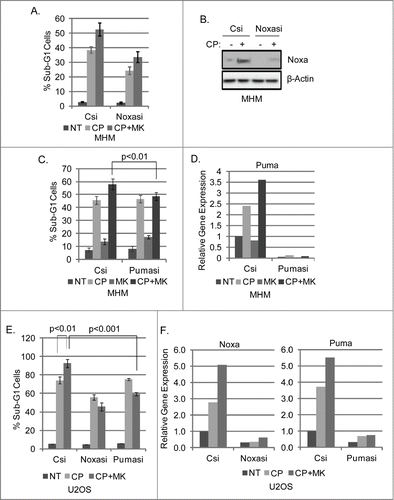
Figure 6. MK2206 causes a G1 arrest/delay in CP treated cells. MG63 cells (A), SAOS cells (B), and U2OS cells (C) were treated with CP (5 μM for MG63, 2 μM for SAOS, 15 μM for U2OS) or CP plus MK2206 (MK, 10 μM). The cells were harvested at the indicated time points and analyzed by FACS for cell cycle. Representative cell cycle profile histograms are shown. The tables below the histograms show the average percent G1, S, and G2/M populations from triplicate experiments +/− s.e.m.
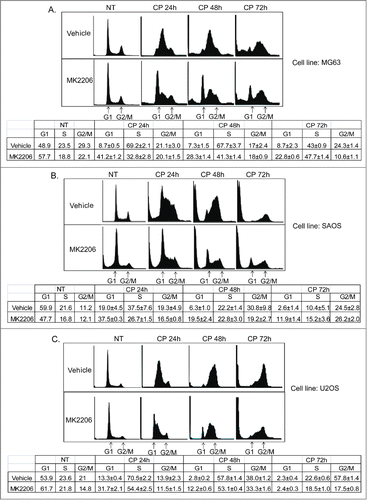
Figure 7. CP induces loss of p27 which is prevented by MK2206. Knockdown of p27 reduces MK2206-mediated G1 arrest and sensitized p53-null cells to apoptosis in response to CP plus MK. (A) MG63, SAOS, U2OS cells were treated with CP and/or MK2206 for 24 hours. Whole cell lysates were immunoblotted for p27 and β-actin. (B) MG63 cells were transiently transfected with control siRNA (Csi) or p27kip siRNA (p27si) and treated with CP (5 μM) and/or MK2206 for 72 hours. The cells were stained by PI and analyzed for cell cycle. Representative cell cycle profiles are shown. The table below the cell cycle profiles shows the average percent G1, S, and G2/M populations from triplicate experiments +/− s.e.m. (C) Upper Percent sub-G1 cell (+/− s.e.m.) is shown for control and p27 knockdown MG63 cells treated with CP, MK, or CP plus MK for 72 hrs. There is significant difference in Sub-G1 and G1 population between Csi and p27si (p < 0.01) cells treated with CP plus MK2206. Lower Immunoblots show efficient knockdown of p27 by siRNA. (D) Upper Percent sub-G1 cell (+/− s.e.m.) is shown for control and p27 knockdown SAOS cells treated with CP, MK, or CP plus MK for 72 hrs. There is significant difference in Sub-G1 and G1 population between Csi and p27si (p < 0.01) cells treated with CP plus MK2206. Lower Immunoblots show efficient knockdown of p27 by siRNA.
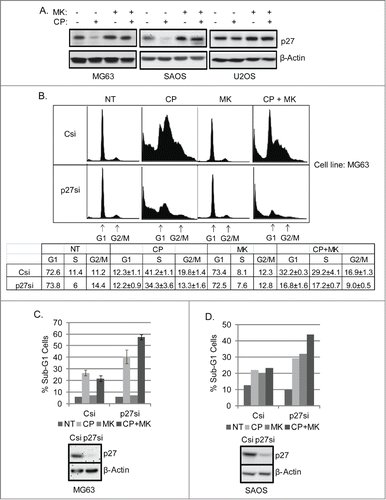
Figure 8. Scheduled inhibition of AKT reduces p27, abrogates G1 arrest, and sensitizes p53-null cells to CP-induced apoptosis. (A) Cells were treated with CP (5 μM for MG63, 2 μM for SAOS) alone, CP plus MK2206 simultaneously (SIM), or CP first for 24 hrs followed by MK2206 (sequentially; SEQ) for 48 hrs. Whole cells lysates were immunoblotted for p27, pAKT, total AKT, and β-actin. (B and C) MG63 and SAOS cells were treated as indicated for 72 hours, and cell cycle determined by FACS. Representative histograms are shown. The tables below the histograms shows the average percent sub-G1, G1, S, and G2/M populations from triplicate experiments +/− s.e.m. There is significant difference in sub-G1 and G1 population (p < 0.01) between CP plus MK (SIM) and CP plus MK (SEQ) in both MG63 and SAOS cells.
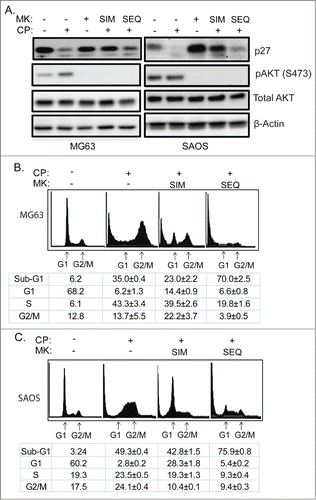
Figure 9. Scheduled inhibition of AKT abrogates G1 arrest/delay, and together with inhibition of Chk1 maximally sensitizes OS cells to CP-induced apoptosis. (A) Cells were treated with CP alone (5 μM for MG63, 2 μM for SAOS, 15 μM for U2OS) or CP plus MK for 48 hrs and immunoblotted for pChk1 (S296 and S345), total Chk1, and β-actin. (B–D) Cells were treated with CP alone (doses as above), or CP plus LY and/or MK either simultaneously (SIM) or sequentially (SEQ). Cells were harvested after 72 hours cell cycle profiles determined. Representative cell cycle histograms are shown. The tables below the histograms shows the average percent sub-G1, G1, S, and G2/M populations from triplicate experiments +/− s.e.m. There is significant difference in sub-G1 and G1 population (p < 0.01) between CP plus LY (SIM) and CP plus LY/MK (SIM), and between CP plus LY/MK (SIM) and CP plus LY/MK (SEQ) in MG63 and SAOS cells. In U2OS cell there is no significant difference in sub-G1 and G1 population (p > 0.5) between CP plus LY (SIM) and CP plus LY/MK (SIM) while there is significant difference (p < 0.01) between CP plus LY/MK (SIM) and CP plus LY/MK (SEQ).
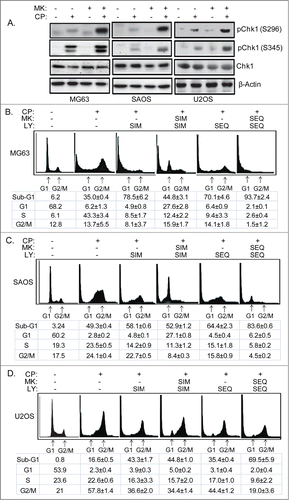
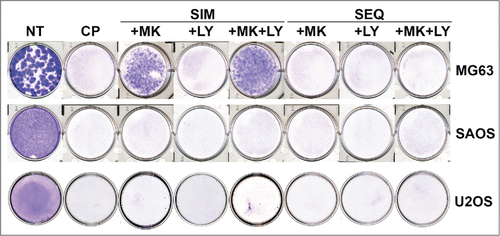
Figure 10. Colony forming ability in OS cells treated with CP, MK2206, and LY either simultaneously or sequentially. U2OS cells were plated at 1.0 × 106 cells/100 mm dish. MG63 and SAOS cells were plated at 1.0 × 105 cells/ well in 6-well dishes, except for the MG63 no treat (NT) condition in which 100 cells were plated. Cells were treated with CP alone (5 μM for MG63, 2 μM for SAOS, 10 μM for U2OS), CP plus 10 μM MK2206, CP plus 0.2 μM LY2603618 or CP plus MK2206 plus LY2603618 eiher simultaneously (SIM) or sequentially (SEQ; MK2206 and LY2603618 added 24 hrs after CP. After 72 hrs, the cells were rinsed 3 times with PBS and then complete media (minus drugs) was added. Cells were allowed to grow for 2 weeks and the plates were then stained with 0.05% Crystal Violet to demonstrate colony formation.