Figures & data
Table 1. VEGFR kinase activity and selectivity profile and bioactivities
Figure 1. Fruquintinib is a highly selective and potent VEGFR1, 2, 3 kinase inhibitor. (A) Chemical structure of fruquintinib. (B) Kinome selectivity of fruquintinib at 1 μmol/L against 253 kinases using Citation32p-ATP incorporation method generated at Millipore. The Kinome tree was downloaded from http://www.cellsignal.com. Percentage (%) denoted the inhibition of fruquintinib at 1 μmol/L to the recombinant kinases. Over 90% inhibition was observed for 3 VEGFR family members (1, 2, 3) and 70~90% inhibition on 4 other kinases, including Fms(Y969C), Ret, and FGFR1 and little effect on remaining kinases tested. IC50s were generated for the kinases of interest and shown in .
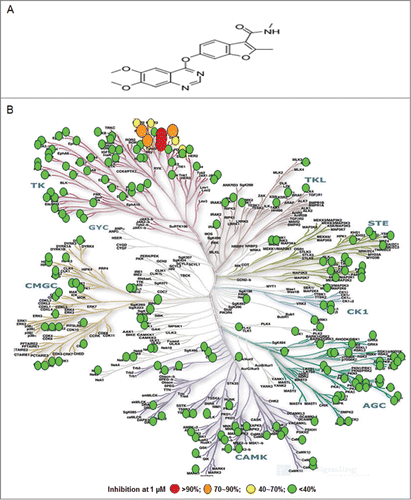
Figure 2. Inhibition on VEGF stimulated activation of KDR and VEGFR3. (A) Fruquintinib inhibited VEGF-A-stimulated KDR phosphorylation and downstream signaling in HUVEC. (b) Fruquintinib abrogated VEGF-A-stimulated KDR phosphorylation and downstream signaling in HEK-293-KDR cell line. (C) Fruquintinib suppressed VEGF-C stimulated VEGFR3 phosphorylation in VEGF-C-stimulated HLEC.
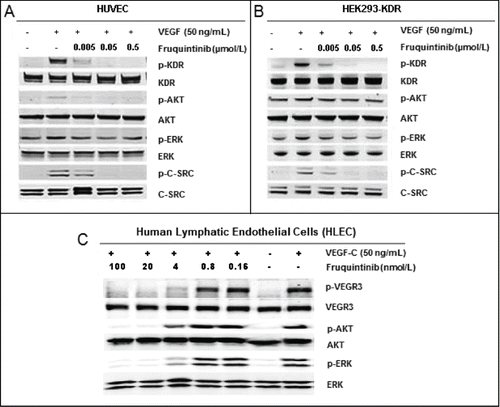
Figure 3. Fruquintinib inhibited HUVEC tubule growth and CAM angiogenesis. Tube formation was suppressed significantly after treatment with fruquintinib at 0.3 μmol/L for 18 hours (A and B) No cytotoxicity was seen at the same concentration of fruquinitib in HUVECs. The plates were incubated for 3 hours at 37°C and fluorescence value was read at Ex 530 nm and Em 590 nm on Tecan (C) Fruquintinib displayed strong inhibition on the development of new blood vessels in the chick embryo (D) Left and right panels, as arrows indicated, were treated with saline and compound, respectively. Pseudolarix acid B was used as a positive control.
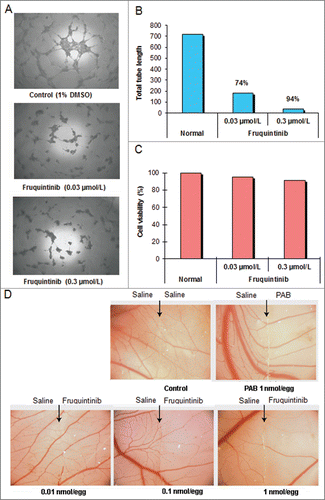
Figure 4. Fruquintinib inhibited p-KDR in lung tissues of mice. (A) Fruquintinib inhibited VEGF-A induced p-KDR in lung tissues. Each group was composed of 3 mice (m1, m2, m3). Animals were treated as described in Method section. (B) Fruquintinib concentration in plasma (purple line) in relation to its inhibition on p-KDR phosphorylation (green bar).
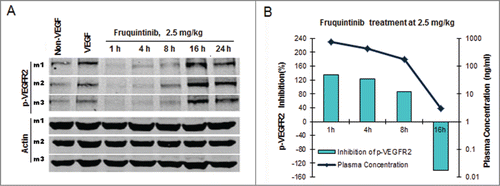
Table 2. Anti-tumor activity of fruquintinib in multiple xenograft tumor models
Figure 5. Fruquintinib inhibited BGC-823 and Caki-1 tumor growth and anti-angiogenesis in tumor tissues. (A) Dose-dependent tumor growth inhibition in BGC-823 tumor following once daily oral treatment of fruquintinib at 0.5, 1 and 2 mg/kg. (B) Anti-tumor effect of fruquintinib at 2, 5 and 20 mg/kg in BGC-823. BGC-823 tumor was regressed by fruquintinib at 5 and 20 mg/kg. (C) Plasma exposures of fruquintinib at 2, 5 and 20 mg/kg in BGC-823 tumor bearing mice following consecutive oral daily dosing. The red line indicated the plasma EC85 for p-KDR inhibition in nude mouse. (D) Dose-dependent tumor growth inhibition in Caki-1 tumor model. (E) CD31 immunohistochemistry staining in Caki-1 xenografts. The pictures were taken at 200× magnification under microscope. Vascular vessels were stained as brown, representing the tumor angiogenesis. (E) Semi-quantification of IHC image. The percentage of CD31 positive area to tumor area was analyzed with NIKON BR-3.0 software. *P < 0.05, **P < 0.01 compared with vehicle group.
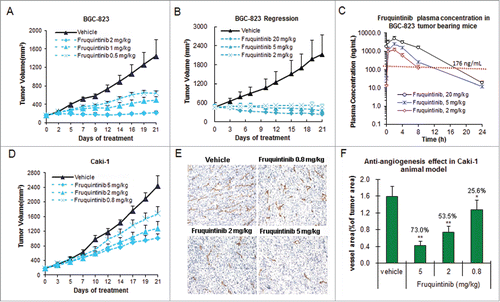
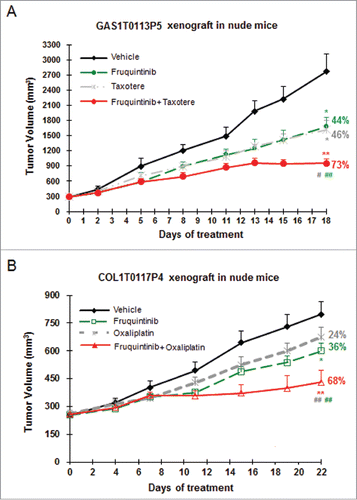
Figure 6. Combination of fruquintinib with chemo drugs shows enhanced anti-tumor effect in PDX models. (A) Fruquintinib in combination with taxotere (docetaxel) in gastric cancer PDX model. (B) Fruquintinib in combination with oxaliplatin in colon cancer PDX model. Percentage (%) represented tumor growth inhibition (TGI) rate. Two-tailed t-test for fruquintinib treatment versus the control group: *, P < 0.05; **, P < 0.01. The combination vs. single agent: #, P < 0.05; ##, P < 0.01.