Figures & data
Table 1. Chemical structures of maritoclax derivatives bearing a pyoluteorin motif. Viability of U937 cells treated with the indicated compounds over 48 hours were used to calculate the EC50 values. For additional compounds that deviate from the pyoluteorin motif, see Table S1
Table 2. The EC50 values of the indicated cell lines treated with the indicated compounds over 48 hours, *28 hours, **24 hours
Figure 1. Pyoluteorin derivatives bind to and induce Mcl-1 degradation. (A) Plot of average chemical shift differences in the spectra of 15N-labeled Mcl-1 upon titration with KS04 (top) and KS18 (bottom). (B) Pockets p2 (left, light blue) and p4 (left, dark red) are indicated on the NMR structure of Mcl-1 complexed with Noxa. The same NMR structure of Mcl-1 is also indicated with residues which demonstrate significant average chemical shift differences according to NMR spectroscopy for all of maritoclax, KS04, and KS18 (right, blue), both KS04 and KS18 (right, red), KS04 alone (right, light green), and KS18 alone (right, dark green). (C) The structure of KS04 and KS18 computationally docked to Mcl-1 by GLIDE. Blue and red colors of the molecular surface represent positive and negative potentials respectively. The carbon atoms of KS04 are colored gray and those of KS18 green. Chlorine atoms are colored dark green, bromine atom purple, oxygen atoms red, nitrogen blue, and polar hydrogen white. Non-polar hydrogen atoms are not shown. Left: The pyrrole group of the KS compounds docked in the p4 pocket of the molecular surface of Mcl-1 with their phenol group extended to the tail region of Mcl-1 helix 8. Right: Hydrogen bonding and amino acid residues near the predicated binding site of KS compounds. Mcl-1 backbone is represented by red to purple ribbons from N-terminus to C-terminus. Two hydrogen bonds between KS compounds and Mcl-1 are shown as yellow dotted lines. (D) U937 cells were treated with vehicle, 2.5 μM KS04 or 2 μM KS18 for 1 hour before adding 10 μg/ml CHX and collecting cells at the indicated times. Protein levels were detected by immunoblotting and quantified by densitometry.
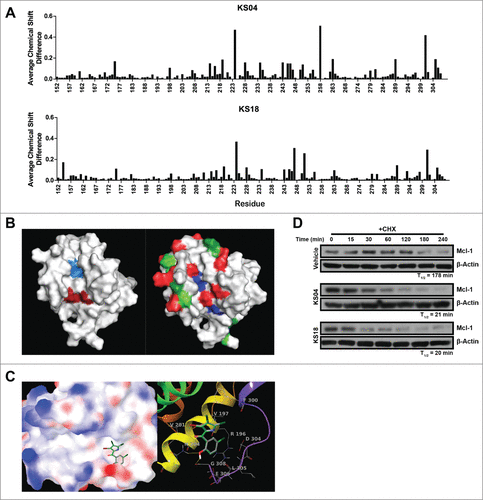
Figure 2. Pyoluteorin derivatives induce Mcl-1-dependent cell death through Bax and Bak in hematological cancer cell lines. Viabilities of K562/Mcl-1-IRES-Bim and K562/Bcl-2-IRES-Bim cells were measured after treatment with the indicated concentrations of KS04 (A) and KS18 (B) for 48 hours. Error bars = SD of 3 replicates. (C) Parental and ΔBak Jurkat cells were treated with the indicated concentrations of KS18 for 48 hours. (D) K562/Mcl-1-IRES-Bim and K562/Bcl-2-IRES-Bim cells were treated with 2 μM KS04 over the indicated times be analyzed through immunoblotting. K562/Mcl-1-IRES-Bim cells were treated with the indicated concentrations of KS18 (E) and KS24 (F) for 18 and 24 hours respectively and analyzed by immunoblotting.
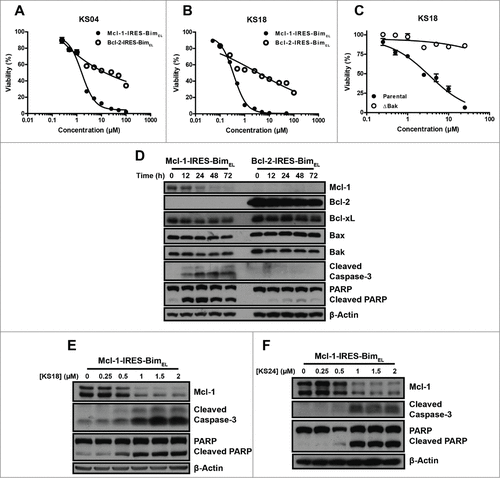
Figure 3. KS04 and KS18 overcome Mcl-1-mediated ABT-737 resistance in hematological cancer cell lines. (A) HL60/ABTR cells were treated with 2 μM KS04 and 1.5 μM KS18 over the indicated times and collected for analysis through immunoblotting. (B) Viability (top) of KG1a/ABTR cells was measured after treatment with the indicated concentrations ABT-737 with vehicle or a sub-optimal concentration of 1.6 μM KS04 for 48 hours. Error bars = SD of 3 replicates. Combination index between ABT-737 and KS04 was calculated based on viability data (bottom). Combination index <1 signifies synergy. (C) HL60/ABTR cells were treated with the indicated concentrations of ABT-737, KS18, or co-treatment at 1:1 ratio to the final indicated concentration over 48 hours to measure viability (top). Error bars = SD of 3 replicates. Combination index between ABT-737 and KS18 was calculated based on the viability data (bottom). (D) HL60/ABTR cells were treated with the indicated concentrations of KS18, ABT-737, or co-treatment at 1:1 ratio to the final indicated concentration for 48 hours and subjected to immunoblotting.
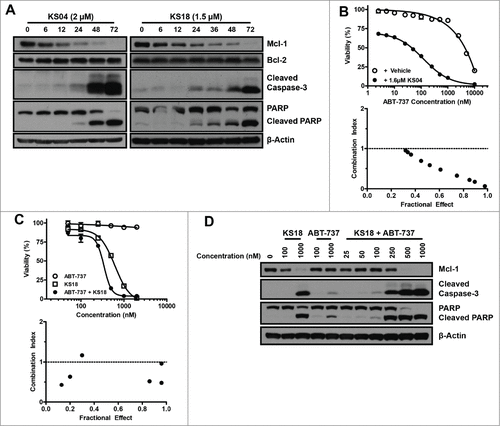
Figure 4. KS04 and KS18 are less toxic to healthy bone marrow cells compared to daunorubicin. (A) U937-luc cells alone or co-cultured with HS-5 stromal cells, or HS-5 stroma cells alone, were treated with the indicated concentrations of daunorubicin (left), KS04 (middle), and KS18 (right) over 48 hours. Viability of single culture cells and the viability of U937-luc cells in the co-culture were determined. Error bars = SD of 3 replicates. (B) Primary mouse bone marrow was treated with maritoclax, KS04, KS18, ABT-737, and daunorubicin over 24 hours to measure viability (errors = SEM of 5 independent experiments). (C) Primary mouse bone marrow seeded to methylcellulose medium supplemented with growth factors were treated with the indicated concentrations of KS18 and daunorubicin for 7 days, and the number of haematopoietic cell colonies were counted.
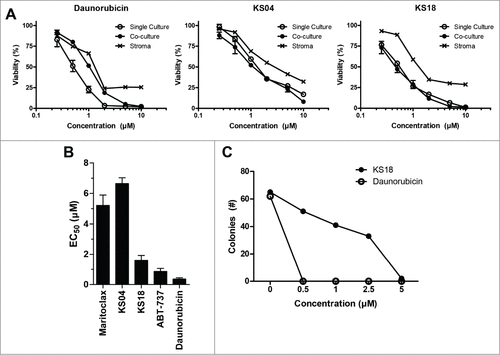
Table 3. The pharmacokinetic parameters of maritoclax and KS18 by intraperitoneal injection in female BALB/c mice
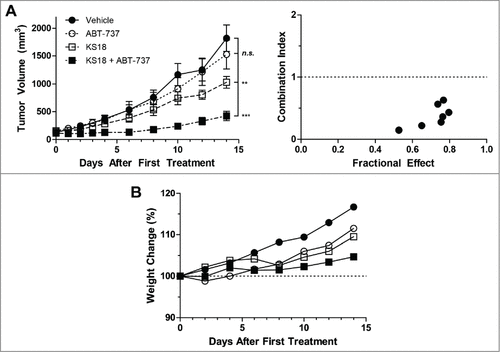
Figure 5. KS18 and ABT-737 synergize to reduce HL60/ABTR xenograft tumor growth in athymic nude mice. (A) Female athymic nude mice bearing HL60/ABTR xenograft tumors were treated with vehicle, ABT-737 (20 mg/kg), KS18 (10 mg/kg), or both ABT-737 and KS18 at 20 mg/kg and 10 mg/kg respectively, for 14 consecutive days after tumor staging. Tumor volumes were measured every 2 days. Error bars = SEM of 10–20 xenograft tumors. n.s. = not statistically significant, **P <0.005, ***P <0.0005 by Student's T-test of tumor volumes at day 14. Combination index between ABT-737 and KS18 was calculated based on perecent tumor size of vehicle control at each day (right). Combination index <1 signifies synergy. (B) The average body weights of mice bearing HL60/ABTR xenograft tumors expressed as a percentage of day 0.