Figures & data
Figure 1. Anti-proliferative activity of cucurbitacin in glioma cell lines and non-neoplastic astrocytes. EGFR overexpressing human glioma cell lines U87-EGFR-WT, U87-EGFRviii and isogenic control, U87 (A), primary cultured glioma cells, GBM-1, GBM-8, GBM-9 and GBM-13 (B) and non-neoplastic human astrocytes (C) were grown on 96-well plates in growth medium and, after an overnight attachment period, were exposed to selected concentrations of inhibitor or vehicle (0, DMSO) for 24, 48, or 72 h (Day 1, Day 2 or Day 3). Cell proliferation inhibition was assessed semiquantitatively by spectrophotometric measurement of MTS bioreduction. Points represent the mean of 3 measurements carried out in triplicate.
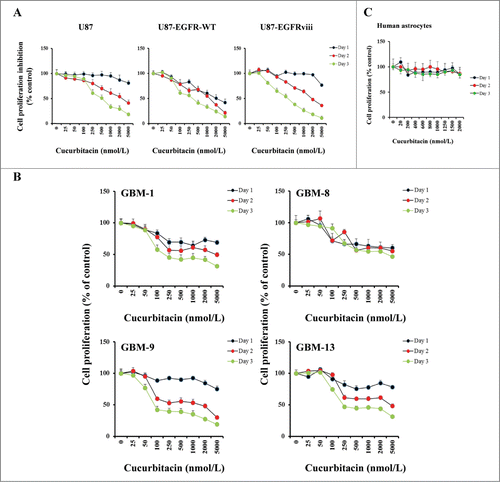
Figure 2. Cucurbitacin inhibits Aurora kinase A, Aurora kinase B and survivin. U87 and U87-EGFRviii (A and B) cells were treated with cucurbitacin (indicated concentration) for 24 h. (C), A panel of 4 glioma cell lines (U87, LN229, T98G and LNZ308) along with EGFR over expressing U87-EGFR-WT (U87-EGFR) and U87-EGFRviii cells were treated with cucurbitacin (indicated concentration) for 24 h. Cell extracts were subjected to Western blot analysis with indicated antibody. (D). U87 cells were treated with cucurbitacin (indicated concentration) for 24 h, after which total RNA was extracted and Aurora kinase A, Aurora kinase B and survivin mRNA levels were quantified by real time PCR as described in the Materials and Methods.
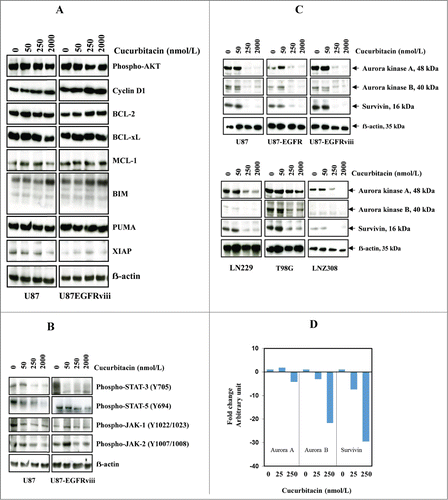
Table 1. The genotypic features of the glioma cell lines used in this study
Figure 3. Cucurbitacin induces G2/M arrest regardless of EGFR/PTEN/p53 status in malignant human glioma cell lines. U87, U87-EGFRviii, T98G, LN18, LNZ308, A172 and LN229 cells were seeded at 60% confluence, allowed to attach overnight, and treated with cucurbitacin (indicated concentration) for 24 h. Control cells received DMSO (0). Cell cycle analysis using propidium iodide (PI) staining was performed as described in the Materials and Methods Section. Representative results of 3 independent experiments are presented here (A). Histogram represents the average of 3 experiments (B). LN18, A172 and LN229 cells were seeded at 60% confluence, allowed to attach overnight, and treated with cucurbitacin (indicated concentration) for 48 or 72 h. Control cells received DMSO (0). Cell cycle analysis was performed as described in the Materials and Methods Section (C).
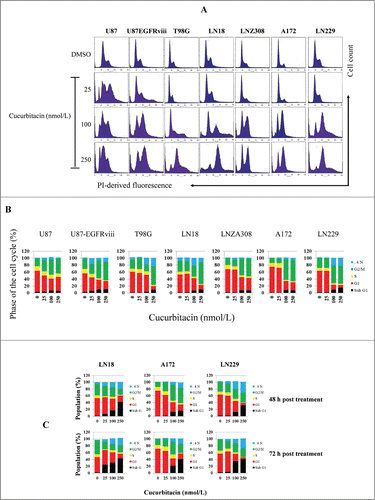
Figure 4. Cotreatment with cucurbitacin and ABT-737 potentiates glioma cell death in a caspase-independent manner. EGFR overexpressing human glioma cell lines U87-EGFR-WT, U87-EGFRviii and isogenic control, U87 (A), A172, LN229 and LNZ308 (B) were seeded at 60% confluence, allowed to attach overnight, and treated with cucurbitacin (C) or ABT-737 (A) or the combination of both (C + A) for 24 h. Control cells received an equivalent amount of DMSO. Apoptosis was analyzed by flow cytometry. The percentages of cells in each quadrant are indicated. (C) Bar chart represents data from 3 independent experiments (** P < 0.005 vs. untreated control or single agent). (D) U87, U87-EGFR-WT and U87-EGFRviii cells were pretreated with pan-caspase inhibitor zVAD-fmk (25 μmol/L) for 2 h followed by combination of cucurbitacin plus ABT-737 (C + A) for 24 h. Cell death was analyzed by flow cytometry. Data are representative of triplicate studies from 3 independent experiments (NS, not significant). U87 and U87-EGFRviii (E) LN229, A172 and LNZ308 (F) were seeded at 60% confluence, allowed to attach overnight, and treated with cucurbitacin (indicated concentration) with or without ABT-737 (500 nmol/L) for 24 h. Control cells received an equivalent amount of DMSO. Cell extracts were subjected to Western blot analysis with indicated antibody. LN18 treated with TRAIL (25 ng/ml for 3 h) served as positive control. G. U87, U87-EGFR-WT, U87-EGFRviii and LNZ308 cells were grown on chamber slides in growth medium, and, after an overnight attachment period, were exposed to cucurbitacin (C, 100 nmol/L) or ABT-737 (A, 1.0 μmol/L) or the combination of both (C + A) or vehicle (DMSO) for 72 h. Cells were washed once with PBS, fixed with 3.7% formaldehyde for 30 min and stained with DAPI for 2 h at room temperature. The slides were then washed in PBS, mounted, and examined under a fluorescent microscope. Cells with multi-lobed nuclei are presented in the bar graph. Points represent the mean of 3 measurements ± S.D (**, P < 0.005).
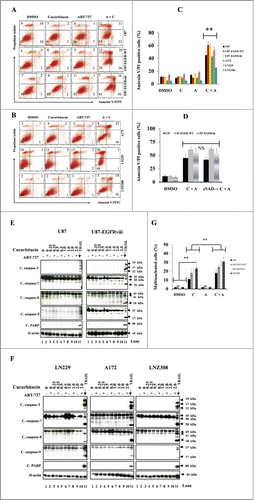
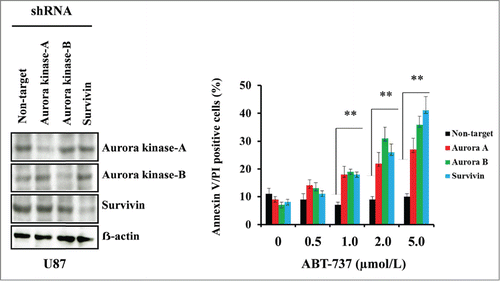
Figure 5. Targeting Aurora kinase A, Aurora kinase B and survivin by RNA interference enhanced ABT-737-induced cytotoxicity. U87 cells were transfected with non-target or Aurora kinase A, Aurora kinase B or survivin shRNA as described in the Materials and Methods. Forty-eight hours post-transfection, cells were treated with the indicated concentrations of ABT-737 for 24 h, and viability was assessed by annexin V/PI apoptosis assay (right panel; ** P < 0.005 versus non-target shRNA control). In parallel, cell lysates were collected and protein was subjected to Western blot analysis using indicated antibodies. Immunoblots were stripped and reprobed with β-actin (left panel). Data are representative of triplicate studies from 3 independent experiments (**, P <0.005).