Figures & data
Figure 1. E2F1 and E2F2 mRNA and protein levels increase following DNA damage in neuronal cells. (A and B) Northern blot analysis of SH-SY5Y cells treated with NCS, H2O2 or UV and harvested at the specified times. Total RNA was extracted from cells and subjected to Northern blot with the [32P]-labeled probes shown in the left margin. In (B), cells were pre-incubated 3 h with 1 μM actinomycin D (Act D). The numbers under the bands indicate E2F1–5 quantitation normalized to β-tubulin and control in (A), and E2F1 and E2F2 quantitation normalized to β-tubulin and None (-) condition in (B). (C) Western blot of E2F1 and E2F2 in SH-SY5Y cells treated with NCS, H2O2 or UV and harvested at the indicated times. The numbers under the bands indicate E2F1 and E2F2 quantitation normalized to β-actin and control. (D–F) Immunoblot of GFP in SH-SY5Y cells expressing E2F1-GFP (D), E2F2-GFP (E) or pEGFP-C1 empty vector (F) and harvested at the indicated times post-UV. (G and H) Western blot of E2F1 and E2F2 in SH-SY5Y cells pre-incubated 3 h with 10 μM cycloheximide (CHX) and harvested at the specified times after genotoxic treatment, as shown in (G). In (D–F, H), data represent the mean±S.E.M. of at least 4 independent experiments for (D and E) and n = 3 for (F and H). In (D–F), P-values were calculated by one-way ANOVA, Dunnett's: *P < 0.05, **P < 0.01, n.s. not significant. C, control mock-treated cells.
![Figure 1. E2F1 and E2F2 mRNA and protein levels increase following DNA damage in neuronal cells. (A and B) Northern blot analysis of SH-SY5Y cells treated with NCS, H2O2 or UV and harvested at the specified times. Total RNA was extracted from cells and subjected to Northern blot with the [32P]-labeled probes shown in the left margin. In (B), cells were pre-incubated 3 h with 1 μM actinomycin D (Act D). The numbers under the bands indicate E2F1–5 quantitation normalized to β-tubulin and control in (A), and E2F1 and E2F2 quantitation normalized to β-tubulin and None (-) condition in (B). (C) Western blot of E2F1 and E2F2 in SH-SY5Y cells treated with NCS, H2O2 or UV and harvested at the indicated times. The numbers under the bands indicate E2F1 and E2F2 quantitation normalized to β-actin and control. (D–F) Immunoblot of GFP in SH-SY5Y cells expressing E2F1-GFP (D), E2F2-GFP (E) or pEGFP-C1 empty vector (F) and harvested at the indicated times post-UV. (G and H) Western blot of E2F1 and E2F2 in SH-SY5Y cells pre-incubated 3 h with 10 μM cycloheximide (CHX) and harvested at the specified times after genotoxic treatment, as shown in (G). In (D–F, H), data represent the mean±S.E.M. of at least 4 independent experiments for (D and E) and n = 3 for (F and H). In (D–F), P-values were calculated by one-way ANOVA, Dunnett's: *P < 0.05, **P < 0.01, n.s. not significant. C, control mock-treated cells.](/cms/asset/d72e765c-b285-457d-8031-0c803c3b42bb/kccy_a_985031_f0001_oc.gif)
Figure 2. DNA damage induced E2F1 and E2F2 are transcriptionally active. (A) CAT activity of Neuro-2a cells transfected with pE2F-CAT or pΔE2F-CAT along with pCEFL-β-galactosidase, and harvested 24 h post-genotoxic treatment. (B and C) CAT activity of Neuro-2a cells transfected with pE2F-CAT, pCEFL-β-galactosidase and 1 μM of the indicated ODN, and harvested 24 h after DNA damage. In all cases, CAT activity was normalized to β-galactosidase activity. In (B and C), results are expressed relative to None-ASLUC or None-mut E2F DO conditions. Data represent the mean±S.E.M. of 3 independent experiments performed in triplicate. P-values were obtained using one-way ANOVA with Tukey's posttest in (A), one-way ANOVA with Dunnett's posttest in (B) and Student's t-test in (C): *P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant. DO, decoy oligodeoxynucleotide.
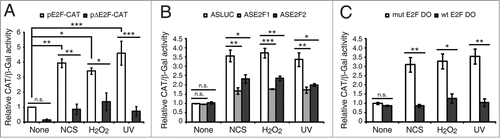
Figure 3. E2F1 and E2F2 transcriptional upregulation requires ATM/ATR and MEK kinases activity. SH-SY5Y cells incubated 1 h with 5 mM caffeine, 10 μM KU-55933, 10 μM PD-98059, 50 μM LY-294002 or 25 μM SP-600125 and harvested after a 4 h treatment with NCS, H2O2 or UV. Total RNA was extracted and subjected to Northern blot analysis with the [32P]-labeled probes shown in the left margin. The numbers under the bands indicate E2F1 and E2F2 quantitation normalized to β-tubulin and None (-) condition.
![Figure 3. E2F1 and E2F2 transcriptional upregulation requires ATM/ATR and MEK kinases activity. SH-SY5Y cells incubated 1 h with 5 mM caffeine, 10 μM KU-55933, 10 μM PD-98059, 50 μM LY-294002 or 25 μM SP-600125 and harvested after a 4 h treatment with NCS, H2O2 or UV. Total RNA was extracted and subjected to Northern blot analysis with the [32P]-labeled probes shown in the left margin. The numbers under the bands indicate E2F1 and E2F2 quantitation normalized to β-tubulin and None (-) condition.](/cms/asset/9614f505-b41f-4d6f-be81-11ed36bec98b/kccy_a_985031_f0003_b.gif)
Figure 4. E2F1 and E2F2 upregulation reduces γH2AX intensity following UV irradiation. (A) SH-SY5Y cells expressing E2F1-GFP, E2F2-GFP or pEGFP-C1 empty vector, fixed 30 minutes post-UV and immunostained with anti-γH2AX antibody. Nuclei were visualized with DAPI staining. Scale bar, 10 μm. (B and C) Percentage of damaged cells obtained by measurement of γH2AX intensity levels. Quantifications were carried out classifying cells according to the E2F expression level: no E2F, low E2F or high E2F in (B), or to the GFP expression level: no GFP, low GFP or high GFP in (C). Results are expressed relative to mock-treated no E2F condition in (B) or mock-treated no GFP condition in (C), which represent the 10% of the maximum γH2AX intensity detected, and UV treatment was normalized to mock-treatment for each of the E2F (B) or GFP (C) intensity levels. Data represent the mean±S.E.M. of at least 4 independent experiments, in which 250 to 400 cells were analyzed for each condition. P-values were calculated by one-way ANOVA, Tukey's: **P < 0.01, ***P < 0.001, n.s. not significant. (D) Immunoblot of E2F1 or E2F2 in SH-SY5Y cells transfected with E2F1-GFP or E2F2-GFP respectively, and in control not transfected (NT) cells. Cells were harvested at the indicated times post-UV. E2F bands correspond to the endogenous protein in NT and to the exogenously expressed protein in E2F-GFP. The numbers under the bands indicate E2F1 and E2F2 quantitation normalized to β-actin and NT-None condition.
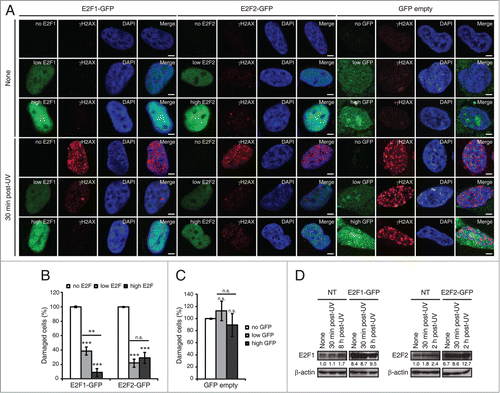
Figure 5. Blockade of E2F1 and E2F2 induction increases γH2AX intensity and reduces DNA repair capability in response to DNA damage. (A–E) Neuro-2A cells transfected with 1 μM of the indicated ODN, exposed to NCS, H2O2 or UV (A) for 4 h (B and D) or 10 h (C and E), fixed and immunostained using anti-γH2AX antibody. Nuclei were visualized with DAPI staining. Scale bar, 10 μm. In (B–E), data represent the mean±S.E.M. of at least 3 independent experiments, in which 300 to 1000 cells were analyzed for each condition. The percentage of damaged cells was obtained by measurement of γH2AX intensity levels. Quantifications were carried out so that data is expressed relative to the control ODNs ASCAT or mut E2F DO, which represent the 10% of the maximum γH2AX intensity detected. P-values were obtained by one-way ANOVA followed by Dunnett's posttest in (B and C) and Student's t-test in (D and E): *P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant. (F) SH-SY5Y cells transfected with 1 μM of the indicated ODN, UV-irradiated and harvested immediately (0 h) or at 6, 24 or 48 h post-irradiation. Genomic DNA was slot-blotted and analyzed by immunoblot for CPD photoproducts. Methylene Blue staining for total DNA was used as a loading control. The table indicates the average of 2 independent experiments of the percentage of remaining CPD photoproducts, obtained by CPD quantitation and normalization to total DNA. DO, decoy oligodeoxynucleotide.
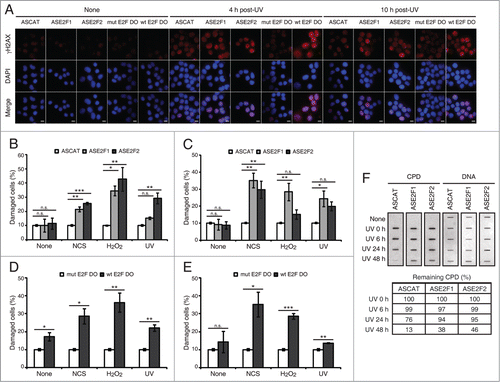
Figure 6. E2F1 and E2F2 reduce apoptotic response after genotoxic stress. SH-SY5Y cells transfected with 1 μM of the specified ODN and treated with H2O2. (A and B) Cell lysates examined for caspase-3 activity 24 h post-H2O2. (C) Western blot of anti-cleaved caspase-3 8 h post-H2O2. The numbers under the bands indicate cleaved caspase-3 quantitation normalized to β-actin and control ODN. In (A and B), data represent the mean±S.E.M. of 4 independent experiments performed in duplicate. One-way ANOVA, Tukey's: *P < 0.05, **P < 0.01, *** P < 0.001, n.s. not significant. DO, decoy oligodeoxynucleotide.
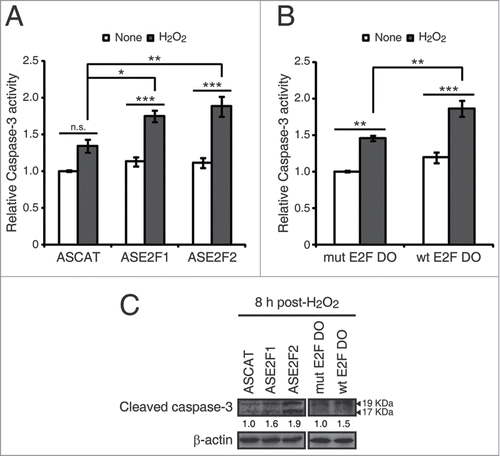
Figure 7. E2F1 and E2F2 confer cellular resistance to genotoxic stimuli. (A and B) Cell survival assessed by MTT reduction assay in SH-SY5Y cells transfected with 1 μM of the specified ODN and exposed to UV irradiation. Data is representative of 4 independent experiments carried out in octuplicate. (C–E) Clonogenic assay in SH-SY5Y cells transfected with 1 μM of the indicated ODN and treated with the DNA damaging agent. In (D and E), results are expressed relative to the control mock-treated cells for each ODN, and data represent the mean±S.E.M. of 4 independent experiments performed in cuadruplicate. P-values were calculated using one-way ANOVA with Dunnett's posttest in (D) and Student's t-test in (E): *P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant. DO, decoy oligodeoxynucleotide.
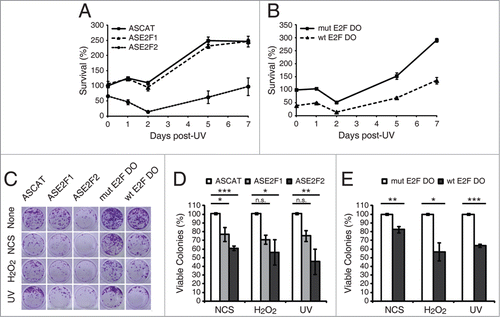
Figure 8. Accumulation of E2F1 and E2F2 at sites of DNA damage. (A) E2F1 and E2F2 Western blot of cytoplasmic and chromatin fractions of SH-SY5Y cells treated with genotoxic agents for the indicated times. GAPDH and H3 were used as cytoplasmic and chromatin specific markers respectively. Data represents the cytoplasm and chromatin-associated E2F relative percentages for each condition, obtained by normalization to GAPDH and H3 correspondingly. (B–E) Live-cell imaging of SH-SY5Y cells expressing E2F1-GFP or E2F2-GFP microirradiated with a 405 nm laser and pre-incubated or not with the photosensitizer Ro 19–8022 (Ro). In (C and E), data represent the mean±S.D. of 2 independent experiments, in which 10 cells were analyzed for each condition. Arrows indicate the site of microirradiation. Scale bar, 2 μm. (F) SH-SY5Y cells expressing E2F1-HA or E2F2-HA (upper panel) or JNK-HA (lower panel) were UV-irradiated or mock-treated, fixed, lysed and ChIP was carried out with anti-HA antibody. Pulled-down DNA was slot-blotted and analyzed by immunoblot for CPD photoproducts. Methylene Blue staining for total DNA was used as a loading control. (G) Co-immunoprecipitation assays of whole-cell lysates from SH-SY5Y cells harvested 1 h post-NCS treatment. Immunoprecipation (IP) was performed with anti-E2F1, anti-E2F2 and anti-E2F4 antibodies and associated proteins were detected by immunoblot (IB). Non specific IgG isotype antibody served as IP control.
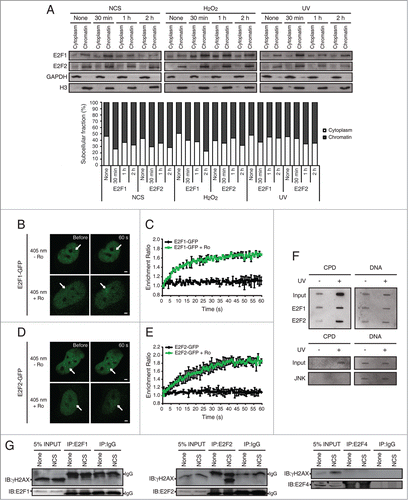
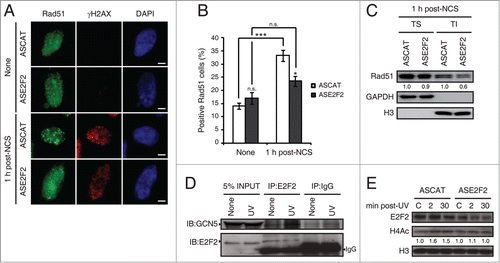
Figure 9. E2F2 promotes Rad51 foci formation and induces histone acetylation in response to DNA damage. (A and B) SH-SY5Y cells transfected with 1 μM of ASE2F2, fixed 1 h post-NCS treatment and immunostained using anti-Rad51 and anti-γH2AX antibodies. Nuclei were visualized with DAPI staining. Scale bar, 10 μm. In (B) data represent the mean±S.E.M. of 4 independent experiments, in which 100 to 250 cells were analyzed for each condition. P-values were calculated using one-way ANOVA with Tukey's posttest: *P < 0.05, *** P < 0 .001, n.s. not significant. Cells with 5 or more Rad51 foci were considered as positive Rad51 cells. (C) Rad51 immunoblot of Triton soluble (TS) and insoluble (TI) fractions of SH-SY5Y cells transfected with 1 μM of ASE2F2 and harvested 1 h post-NCS treatment. GAPDH and H3 were used to detect soluble cytoplasmic and chromatin-bound proteins respectively. The numbers under the bands indicate Rad51 quantitation normalized to GAPDH or H3 in TS or TI fractions correspondingly, and ASCAT condition for each fraction. (D) Co-immunoprecipitation assay of whole-cell lysates from SH-SY5Y cell harvested 30 minutes post-UV irradiation. Immunoprecipation (IP) was performed with anti-E2F2 antibody and associated proteins were detected by immunoblot (IB). Non specific IgG isotype antibody served as IP control. (E) Western blot of acetylated H4 (H4Ac) in SH-SY5Y cells transfected with 1 μM of ASE2F2 and harvested 2 or 30 minutes following UV light exposure. The numbers under the bands indicate H4Ac quantitation normalized to H3 and ASCAT-control condition. C, control mock-treated cells.