Figures & data
Figure 1 (See previous page). Tctex1d2 and Wdr60 associate with cytoplasmic dynein complex 1 and 2. (A) Schematic of Tctex1 domain containing proteins. All members have a carboxyl terminal Tctex1 domain and a variable N- terminal domain implicated in cargo binding. The number of amino acid residues is indicated for each protein. (B) LAP-Tctex1d2 tandem affinity purification. MW= molecular weight marker, HSS= high spin supernatant, E= final eluates. Asterisk denotes Tctex1d2. (C) Cytoscape analysis showing that Tctex1d2 interacting proteins can be grouped into 6 major categories: tubulin isoforms, microtubule binding proteins, centrosome associated proteins, proteins associated with ciliogenesis, cytoplasmic dynein complex 1 and 2 (Dync1 and Dync2) subunits, and proteins implicated in other cellular roles. (D) Summary of mass spectrometry data for Dync1 and Dync2 components identified in the LAP-Tctex1d2 purification, including protein name, number of peptides identified, number of unique peptides, and the percent protein coverage. (E) HEK293 or HEK293 LAP-Tctex1d2 expressing cell extracts were used to perform immunoprecipitations (IPs) with anti-GFP antibodies. IPs were resolved by SDS PAGE, transferred to a PVDF membrane, and immunoblotted with indicated antibodies. (F and G) Reciprocal co-IPs of endogenous Tctex1d2 and Wdr60 from HEK293 cells, using anti-Tctex1d2 or anti-Wdr60 antibodies. Western blot analysis shows that Tctex1d2 and Wdr60 co-IP with each other and both IP Dync1 and Dync2 subunits. See also Fig. S1 and Table S1. (H) In vitro binding assays testing the binding of in vitro transcribed/translated 35S-radiolabeled HA-tagged full-length Tctex1d2, N-terminal Tctex1d2 (amino acids 1-40), and C-terminal Tctex1d2 (amino acids 41-142) with FLAG-tagged full-length Wdr34 and Wdr60. Binding was detected by radiometric analyses. I indicates Tctex1d2 input only, numbers 1-3 indicate incubation with Wdr60 (1), Wdr34 (2), or Luciferase (3).
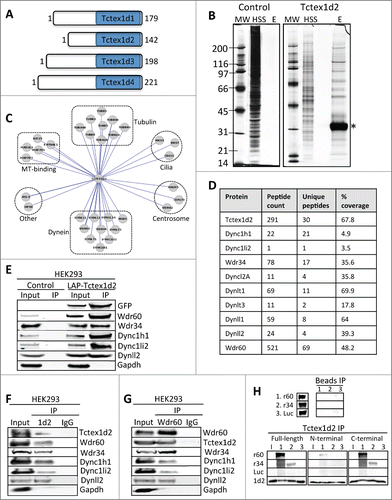
Figure 2 (See previous page). Tctex1d2 and Wdr60 localize to microtubule organizing centers. (A and B) Immunofluorescence microscopy of fixed HeLa cells stained with Hoechst 33342 DNA dye, and anti-α-tubulin, anti-Tctex1d2 (A), or anti-Wdr60 (B) antibodies. Images show the cell cycle subcellular localization of Tctex1d2 (A) and Wdr60 (B). Note that both proteins localize to the microtubule-organizing center (MTOC) in interphase, the spindle poles during mitosis (prometaphase, metaphase) and cytokinesis, and near the cytokinetic bridge during cytokinesis. Bar= 5 μm. (C and D) Immunofluorescence microscopy of fixed interphase HeLa cells stained with Hoechst 33342 DNA dye, anti-γ-tubulin, anti-Centrin, anti-Tctex1d2 (C), or anti-Wdr60 (D) antibodies. Bar= 5 μm. Lower panels show a zoom view of the MTOC region. Bar= 2 μm. (E and F) Immunofluorescence microscopy of fixed interphase HEK293 cells expressing LAP-Tctex1d2 fixed and stained with Hoechst 33342 DNA dye, anti-α-tubulin, anti-PCM1 (E), or anti-Wdr60 (F) antibodies. Bar= 5 μm. Lower panels show a zoom view of the MTOC region. Bar= 2 μm. (G and H) Immunofluorescence microscopy of fixed interphase HeLa cells treated with or without Nocodazole, fixed, and stained with Hoechst 33342 DNA dye, anti-γ-tubulin, anti-α-tubulin, anti-Tctex1d2 (G), or anti-Wdr60 (H) antibodies. Bar= 5μm. Rightmost panels show a zoom view of the MTOC region. Bar= 2 μm. (I) Analysis of Tctex1d2 and Wdr60 protein levels throughout the cell cycle. HeLa cells were synchronized in G1/S, released into the cell cycle and cells were harvested at the indicated time points. Protein extracts were prepared, resolved by SDS PAGE, transferred to a PVDF membrane and immunoblotted with indicated antibodies. Note that Tctex1d2 and Wdr60 protein levels remain constant throughout the cell cycle. See also Fig. S2.
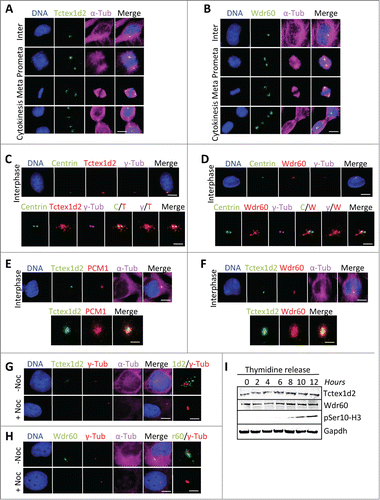
Figure 3. Tctex1d2 and Wdr60 associate with cytoplasmic dynein complex 2 and localize to the base of the cilia in ciliated cells. (A) Cytoscape analysis showing that Tctex1d2 interacts with the Dync2 complex along with tubulin isoforms, microtubule binding proteins, centrosome associated proteins, proteins associated with ciliogenesis, and proteins implicated in other cellular roles in ciliated cells. (B) Summary of mass spectrometry data for Dync2 subunits identified in the LAP-Tctex1d2 purification from ciliated HEK293 cells, including protein name, number of peptides identified, number of unique peptides, and percent protein coverage. (C and D) Reciprocal co-IPs of endogenous Tctex1d2 and Wdr60 from ciliated hTERT-RPE cells, using anti-Tctex1d2 or anti-Wdr60 antibodies. Immunoblot analysis shows that Tctex1d2 and Wdr60 co-IP with each other and both IP Dync2 subunits. (E–H) Immunofluorescence microscopy of fixed interphase or ciliated hTERT-RPE cells stained with Hoechst 33342 DNA dye, anti-γ-tubulin, anti-Tctex1d2 (E and F), or anti-Wdr60 (G and H) antibodies. Bar= 5 μm. Lower panels show a zoom view of the MTOC region (E and G) or the base of the cilium (F and H). Bar= 2 μm. (I) Immunofluorescence microscopy of fixed ciliated hTERT-RPE cells expressing LAP-Tctex1d2 showing that Tctex1d2 localizes to the ciliary base and axoneme. Cell staining was as described in (C). Bar= 2 μm.
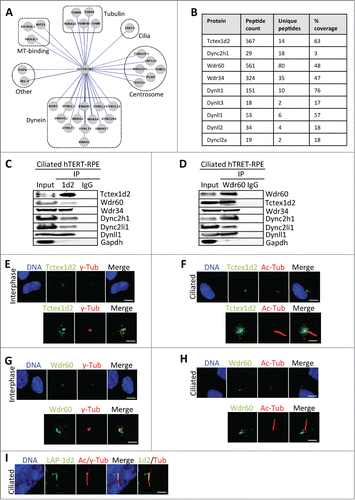
Figure 4. Depletion of Tctex1d2 or Wdr60 leads to ciliation defects. (A and B) siRNA knockdown of Tctex1d2 or Wdr60 protein levels. Immunoblot analysis showing that siRNA pool oligonucleotides targeting TCTEX1D2 (si1D2) or WDR60 (siR60) deplete Tctex1d2 or Wdr60 protein levels in hTERT-RPE cells compared to non-targeting control siRNA (siCont). (C and D) Immunofluorescence of ciliated hTERT-RPE cells treated with siCont, si1D2, or siR60 for 48 hours, starved for 24 hours, and stained with Hoechst 33342 DNA dye, anti-γ-tubulin, anti-acetylated (Ac)-tubulin, and anti-Tctex1d2 (C) or anti-Wdr60 (D). Note the lack of Tctex1d2 at the base of the cilium in si1D2 treated cells and defective cilia formation. Similarly the lack of Wdr60 at the base of the cilium in siR60 treated cells and defective cilia formation. Scale bar = 5 μm. Rightmost panels show a zoom view of the base of the cilium. Bar= 2 μm. (E) Quantitation of the percentage of ciliated cells siCont, si1D2, siR60 and si1D2/siR60-treated cells. Data represent the average ± SD of 3 independent experiments, 100 cells counted for each, p values are as indicated. NS indicates not statistically significant. (F and G) Immunofluorescence of ciliated hTERT-RPE cells treated with siCont, si1D2, or siR60 for 48 hours, starved for 24 hours, and stained with Hoechst 33342 DNA dye, anti-γ-tubulin, anti-acetylated (Ac)-tubulin, and anti-Wdr60 (F) or anti-Tctex1d2 (G). Note Wdr60 localizes to the base of the cilium in si1D2 treated cells, whereas Tctex1d2 is mostly absent from the base of the cilium in siR60 treated cells. Scale bar = 5 μm. Rightmost panels show a zoom view of the base of the cilium. Bar= 2 μm.
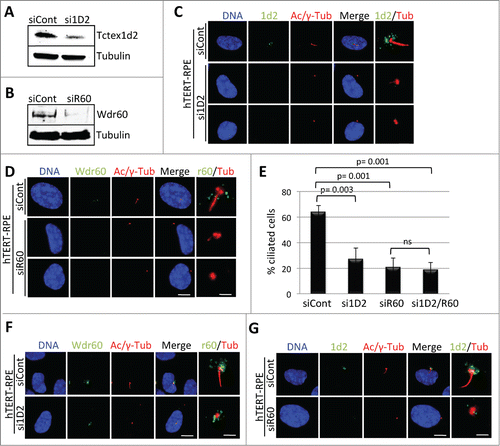
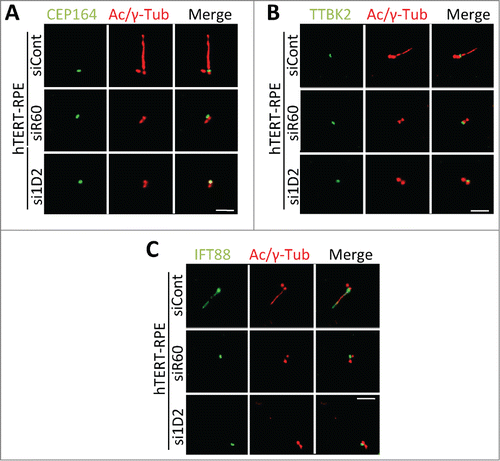
Figure 5. Depletion of Tctex1d2 or Wdr60 does not affect the recruitment of some factors necessary for the early steps of ciliogenesis. (A–C) Immunofluorescence microscopy of ciliated hTERT-RPE cells treated with siCont, si1D2, or siR60 for 48 hours, starved for 24 hours, and stained with Hoechst 33342 DNA dye, anti-γ-tubulin, anti-acetylated (Ac)-tubulin, and anti-CEP164 (A), anti-TTBK2 (B), or anti-IFT88 (C). Note that all proteins localize to the mother centriole in si1D2 and siR60 treated cells. Scale bar = 5 μm.