Figures & data
Figure 1. Increased frequency and length of cilia in senescent human fibroblasts; (A) Doubling time over extended culture periods of BJ, MRC5 and NHDF cells; (B). Immunofluorescence microscopy of senescent BJ cells stained with antibodies to Cep135 (green) and γ-H2AX (red). DNA was visualised with DAPI (blue). Scale bar, 10 μm; (C) Quantitation of the frequency of centrosomal amplification in senescent BJ cells with γ-H2AX staining, scored as >4 centrin2 spots in a cell. Histograms show means ± s.d. of 3 separate experiments in which at least 200 cells were quantitated; (D) Quantitation of the ciliation frequency in the indicated cells, based on imaging of acetylated tubulin. ‘Unt’, untreated. Serum starvation (‘Ser.-stvd’) consisted of 24 h culture with 0.1% newborn calf serum. Histograms show means ± s.d. of 3 separate experiments in which at least 200 cells were quantitated; (E) Immunofluorescence microscopy of the indicated cells stained with antibodies to Cep135 (green) and acetylated tubulin (red). DNA was visualised with DAPI (blue). Scale bar, 10 μm; (F) Quantitation of cilium length in the indicated cell lines. At least 30 ciliated cells were scored for each. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with controls of the same cell line by unpaired t-test.
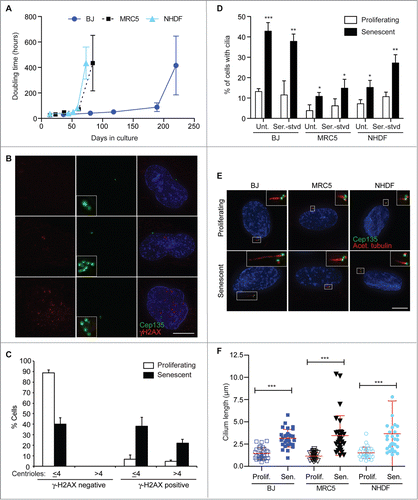
Figure 2. Cell-intrinsic control of extended cilium length in senescent cells; (A) Immunofluorescence microscopy of senescent BJ cells stained with antibodies to Arl13b (green) and γ-H2AX (red). DNA was visualised with DAPI (blue). Scale bar, 10 μm; (B) Quantitation of ciliation frequency in senescent BJ cells with γ-H2AX staining. Histograms show means ± s.d. of 3 separate experiments in which at least 200 cells were quantitated; (C) Immunofluorescence microscopy of senescent BJ cells stained with antibodies to centrin3 (red) and Arl13b (green) or Cep135 (red) and acetylated tubulin (green), as indicated. DNA was visualised with DAPI (blue). Scale bar, 10 μm; (D) Quantitation of cilium length in BJ cells, before or 24 h after the washout of 72 h 4 mM chloral hydrate treatment. This chloral hydrate treatment caused the removal of 90% of cilia from both proliferating and senescent cells. At least 30 ciliated cells were scored for each condition. **, P < 0.01; ***, P < 0.001 in comparison to the indicated controls by unpaired t-test; (E) Immunoblot analysis of CP110 expression in BJ cells. Size markers are indicated in kDa.
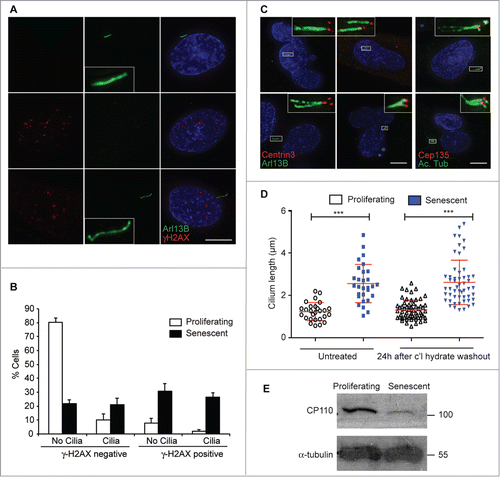
Figure 3. Hedgehog inhibition blocks proliferation and increases cilium length; (A) Immunofluorescence microscopy of BJ cells stained with antibodies to Ki67 (green). DNA was visualised with DAPI (blue). Scale bar, 10 μm; (B) Quantitation of the proliferative index of BJ cells after the indicated treatment, as determined by microscopy analysis of Ki67 signal. Histograms show means ± s.d. of 3 separate experiments in which at least 100 cells were quantitated; (C) Quantitation of cilium length in the indicated cells. At least 30 ciliated cells were scored for each condition, even when there were very few such cells. ***, P < 0.001 in comparison to untreated or indicated controls by unpaired t-test. ‘ns’, not significant; (D) Immunoblot analysis of the indicated protein expression in BJ cells. Size markers are indicated in kDa.
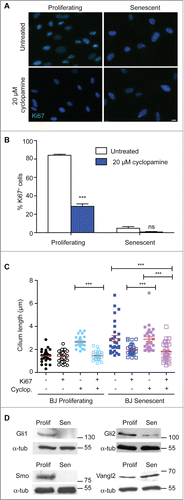
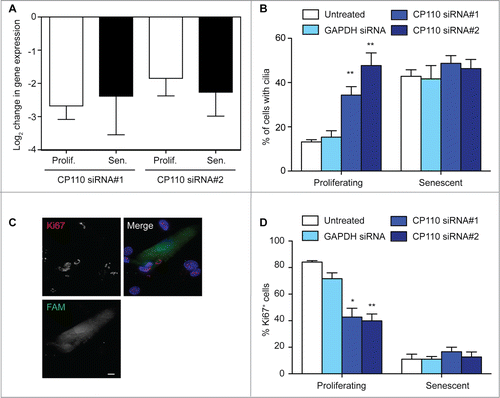
Figure 4. CP110 depletion causes increased ciliation and reduced cellular proliferation; (A) Quantitation of CP110 depletion in BJ cells by siRNA as determined by quantitative RT-PCR. Knockdowns are compared to the effect of GAPDH siRNA normalized to housekeeping gene expression. Data show the mean—s.d. of 3 separate experiments; (B) Quantitation of the ciliation frequency in BJ cells after the indicated treatments. Histograms show means + s.d. of 3 separate experiments in which at least 100 cells were quantitated; (C) Microscopy of CP110 siRNA-transfected cells stained with antibodies to Ki67 (red in merge). Fluorescent RNA (FAM; green) was cotransfected at a ratio of 1:5 with the siRNA as a transfection control. DNA was visualised with DAPI (blue). Scale bar, 10 μm; (D) Quantitation of the proliferative index of BJ cells after the indicated treatment, as determined by microscopy analysis of Ki67 signal. Histograms show means ± s.d. of 3 separate experiments in which at least 100 transfected cells were quantitated; *, P < 0.05; **, P < 0.01 in comparison with GAPDH siRNA controls by unpaired t-test.