Figures & data
Figure 1. Roles of EphB4 and ephrin-B2 in vascular morphogenesis. (A) Dorsal aorta and cardinal vein formation in zebrafish and mouse embryos. In the zebrafish embryo (left), the DA (red, ephrin-B2+) and CV (blue, EphB4+) segregate from a common precursor vessel by ventral migration of venous-fated cells, which requires ephrin-B2a and EphB4a. In early mouse development, CVs form by sprouting of venous-fated (EphB4+) endothelial cells from an early dorsal aorta, which contains precursors with venous or no detectable arteriovenous commitment in addition to ephrin-B2+ arterial cells. (B) In angiogenic sprouting, invasive tip cells, which are motile, lead sprouts and emit filopodia, are followed by more proliferative stalk cells. Ephrin-B2 (red) expression in tip cells and other angiogenic ECs partially overlaps with EphB4 in stalk cells at the sprout base and capillaries (blue). Pericytes (yellow) stabilize the sprout. Following the extension of sprouts, tip cells establish contact with other sprouts or existing vessels. This leads to the formation of a new vessel branch, which subsequently gets lumenized and stabilized by mural cells.
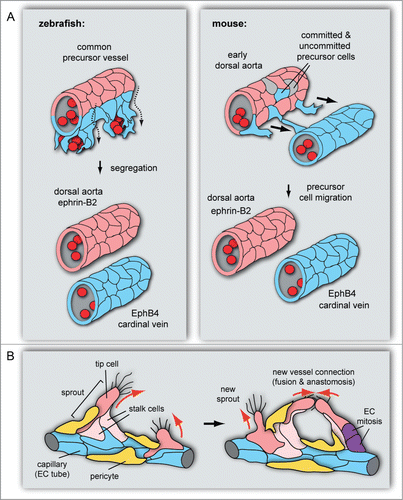
Figure 2. Structure and function of Eph/ephrin molecules. (A) Schematic representation of Eph receptor and ephrin ligand interactions and structural domain organization. Eph/ephrin interaction in trans leads to bidirectional signaling between neighboring cells. The following protein domains and motifs are indicated: ligand binding, cysteine (Cys)-rich, fibronectin (FN) type III, kinase, SAM and PDZ binding domains, transmembrane (TM) region, and tyrosine phosphorylation sites (Y). Binding (arrows) takes place predominantly within the same class: Eph-A receptors bind GPI-anchored ephrin-A ligands and EphBs interact with ephrin-B transmembrane proteins. There are also exceptions for interactions involving proteins from different subclasses (dashed arrows). (B) Eph/ephrin interactions frequently lead to repulsion, which is important for cell migration, cell guidance and cell sorting. The latter can promote cell segregation events that minimize interactions between Eph and ephrin-expressing cells, which facilitates tissue patterning processes during developmental morphogenesis. (C) Eph and ephrin monomers, homo- and heterodimers are inactive. Heterotetramers have weak signaling activity, which is increased by cluster oligomerization and assembly of larger receptor clusters that can expand laterally through Eph–Eph cis interactions that do not require interaction with ephrins.
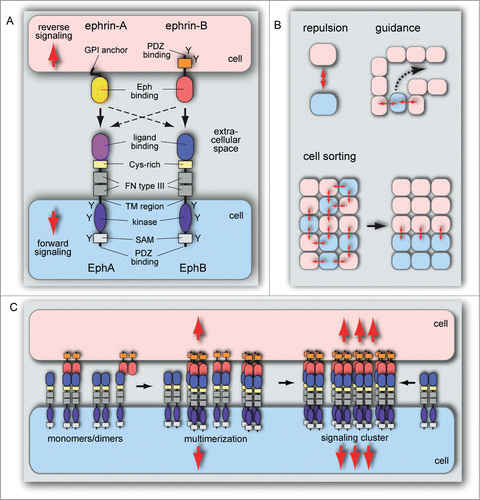
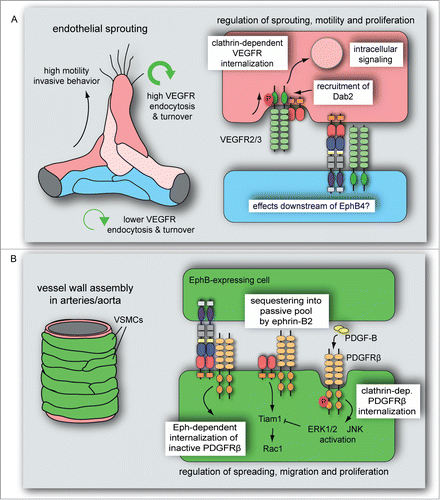
Figure 3. Ephrin-B2 modulates VEGF- and PDGF receptors endocytosis in vascular cells. (A) VEGFR endocytosis and turnover is higher in sprouting cells compared to established vessels. Ephrin-B2 and its interacting partners, the clathrin-associated sorting protein Dab2 and the cell polarity regulator PAR-3, control VEGFR activity, clathrin-dependent VEGFR internalization and downstream intracellular signal transduction. These interactions and activities dynamically regulate vascular endothelial cell sprouting, motility and proliferation. It is currently unknown whether VEGF signaling is also modulated by processes downstream of EphB4. (B) PDGF-B-induced PDGFRβ activation leads to clathrin-dependent PDGFRβ internalization followed by ERK1/2 and JNK activation in VSMCs. This process is limited by ephrin-B2, which sequesters PDGFRβ into a caveolin-dependent, passive pool and positively regulates Tiam1 expression and Rac1 activation. Eph-dependent ephrin-B2 internalization in interacting VSMCs induces the endocytosis of inactive (unphosphorylated) PDGFRβ. PDGF-induced ERK1/2 activation counteracts Tiam1 expression, which reduces VSMC spreading, migration and proliferation.